| CPC C07D 207/277 (2013.01) [C07C 233/60 (2013.01); C07C 301/02 (2013.01); C07D 257/08 (2013.01); C08G 61/08 (2013.01); C08G 2261/136 (2013.01); C08G 2261/1424 (2013.01); C08G 2261/1432 (2013.01); C08G 2261/1646 (2013.01); C08G 2261/354 (2013.01); C08G 2261/418 (2013.01)] | 30 Claims |
|
1. An end-functionalized polymer of Formula (III):
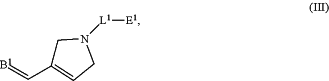 or a tautomer, isotopically labeled polymer, or salt thereof, wherein:
B1 is a brush polymer or star polymer;
L1 is substituted or unsubstituted, C1-1000 alkylene, substituted or unsubstituted, C2-1000 alkenylene, substituted or unsubstituted, C2-1000 alkynylene, substituted or unsubstituted, C1-1000 heteroalkylene, substituted or unsubstituted, C2-1000 heteroalkenylene, or substituted or unsubstituted, C2-1000 heteroalkynylene;
optionally wherein one or more backbone carbon atoms of the C1-1000 alkylene, C2-1000 alkenylene, C2-1000 alkynylene, C1-1000 heteroalkylene, C2-1000 heteroalkenylene, or C2-1000 heteroalkynylene are independently replaced with substituted or unsubstituted carbocyclylene, substituted or unsubstituted heterocyclylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene, as valency permits;
E1 is a thiophile, a first click-chemistry handle, a nucleophile, an electrophile, or a leaving group, H, halogen, substituted or unsubstituted, C1-6 alkyl, substituted or unsubstituted, C2-6 alkenyl, substituted or unsubstituted, C2-6 alkynyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, —ORa, —N(Ra)2, —SRa, —CN, —SCN, —C(═O)Ra, —C(═O)ORa, —C(═O)N(Ra)2, —C(═NRa)Ra, —C(═NRa)ORa, —C(═NRa)N(Ra)2, —NO2, —N3, —NRaC(═O)Ra,—NRaC(═O)ORa,—NRaC(═O)N(Ra)2,—NRaC(═NRa)Ra, —NRaC(═NRa)ORa,—NRaC(═NRa)N(Ra)2, —OC(═O)Ra, —OC(═O)ORa, —OC(═O)N(Ra)2, —OC(═NRa)Ra, —OC(═NRa)ORa, —OC(═NRa)N(Ra)2,—NRaS(═O)2Ra, —NRaS(═O)2ORa, —NRaS(═O)2N(Ra)2, —OS(═O)Ra, —OS(═O)ORa, —OS(═O)N(Ra)2, —S(═O)Ra, —S(═O)ORa, —S(═O)N(Ra)2, —OS(═O)2Ra, —OS(═O)2ORa, —OS(═O)2N(Ra)2, —S(═O)2Ra, —S(═O)2ORa, —S(═O)2N(Ra)2, or —P(═O)(Ra)2; and
each instance of Ra is independently H, substituted or unsubstituted, C1-6 alkyl, substituted or unsubstituted, C2-6 alkenyl, substituted or unsubstituted, C2-6 alkynyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, a nitrogen protecting group when attached to a nitrogen atom, an oxygen protecting group when attached to an oxygen atom, or a sulfur protecting group when attached to a sulfur atom, or two instances of Ra attached to a nitrogen atom are joined with the nitrogen atom to form substituted or unsubstituted heterocyclyl or substituted or unsubstituted heteroaryl.
|