| CPC C07C 247/04 (2013.01) [A61K 31/40 (2013.01); A61K 47/6889 (2017.08); A61P 35/00 (2018.01); C07D 209/20 (2013.01); C07D 403/12 (2013.01); A61K 45/06 (2013.01)] | 19 Claims |
|
1. A compound of the formula:
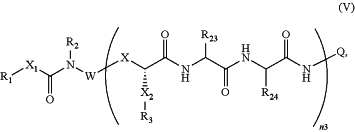 wherein:
X1 is a covalent bond, alkanediyl(C≤12), or substituted alkanediyl(C≤12);
R1 is hydrogen, —(OCH2CH2)n1ZR6, or substituted —(OCH2CH2)n1ZR6, wherein:
n1 is 0-50; and
R6 is hydrogen, hydroxy, amine, mercapto, hydroxylamine, hydrazine, or azide; or
alkyl(C≤12), alkenyl(C≤12), alkynyl(C≤12), alkylhydrazine(C≤12), or a substituted version of any of these groups;
a polyglycine comprising from 1 to 6 glycine units; or
a substructure of the formula:
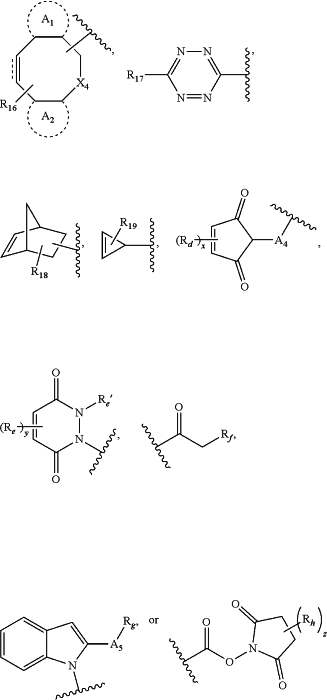 wherein:
A1 and A2 are each independently absent or arenediyl(C≤12), substituted arenediyl(C≤12), heteroarenediyl(C≤12), or substituted heteroarenediyl(C≤12), and form a fused arene(C≤12), substituted arene(C≤12), heteroarene(C≤12), or substituted heteroarene(C≤12);
A4 or A5 are each independently selected from a covalent bond, alkanediyl(C≤8), or substituted alkanediyl(C≤8);
Rd, Re, Re′, and Rh are each independently selected from hydrogen, halo, sulfate, tosylate, mesylate, aryl(C≤8), or substituted aryl(C≤8);
Rf is halo;
Rg is amine, hydrazine, alkylamino(C≤8), substituted alkylamino(C≤8), dialkylamino(C≤8), substituted dialkylamino(C≤8), alkylhydrazine(C≤8), or substituted alkylhydrazine(C≤8);
X4 is O, N, CH2, or X4 is alkanediyl(C≤8) or substituted alkanediyl(C≤8);
R16 is hydroxy, amino, or oxo;
R17 is carboxy; or
alkyl(C≤12), amido(C≤12), aryl(C≤12), heteroaryl(C≤12), —C(O)NR20R20′, or a substituted version of any of these groups wherein:
R20 and R20′ are each independently hydrogen; or
alkyl(C≤12), aryl(C≤12), or a substituted version of either of these groups;
R18 and R19 are each independently hydroxy, amino, halo; or
alkyl(C≤12), aryl(C≤12), or a substituted version of either of these groups;
Z is a covalent bond, alkanediyl(C≤12), —C(O)-alkanediyl(C≤12), —C(O)-alkanediyl(C≤12)-C(O)NH—, or a substituted version of any of these groups;
R2 is hydrogen, alkyl(C≤12), substituted alkyl(C≤12), acyl(C≤12), substituted acyl(C≤12), or a monovalent amino protecting group;
W is a covalent bond or a polyvalent polymer having 3-21 connection points;
n3 is 1 to 20 provided that when W is a covalent bond then n3 is 1 and when W is a polyvalent polymer then n3 is less than or equal to one less than the number of connection points;
each X is independently a covalent bond, alkanediyl(C≤12), or substituted alkanediyl(C≤12);
each X2 is independently alkanediyl(C≤12) or substituted alkanediyl(C≤12);
each R3 is independently hydroxy, or amino; or
alkoxy(C≤12), acyloxy(C≤12), alkylamino(C≤12), dialkylamino(C≤12), amido(C≤12), heteroaryl(C≤12), or a substituted version of any of these groups; or
—X9—C(O)R7, wherein:
X9 is O, —NRb—, or a covalent bond;
Rb is hydrogen, alkyl(C≤6), substituted alkyl(C≤6), or a monovalent amino protecting group;
R7 is hydroxy or amino; or
alkoxy(C≤12), alkylamino(C≤12), dialkylamino(C≤12), or a substituted version of any of these groups; or
-A3SO2NR21R21′, -A3P(O)(OH)OR22, or -A3SO2OR22′, wherein:
A3 is O, —NRc—, or a covalent bond;
Rc is hydrogen, alkyl(C≤6), substituted alkyl(C≤6), or a monovalent amino protecting group;
R21, R21′, R22, and R22′ are each independently hydrogen, alkyl(C≤12), cycloalkyl(C≤12), aryl(C≤12), heteroaryl(C≤12), aralkyl(C≤12), heteroaralkyl(C≤12), or a substituted version of any of these groups;
each R23 is independently the side chain moiety of alanine, valine, leucine, isoleucine, phenylalanine, tyrosine, tryptophan, a hydroxyl-protected version of tyrosine, or an amino-protected version of tryptophan; and
each R24 is independently the side chain moiety of alanine, ornithine, lysine, arginine, citrulline, or an amino-protected version thereof;
each Q is independently a group of the formula:
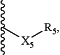 wherein:
each R5 is independently hydrogen or —C(O)—R8, wherein:
R8 is a chemotherapeutic agent;
each X5 is independently a covalent bond, O, S, —NH—, alkanediyl(C≤12), substituted alkanediyl(C≤12), —(OCH2CH2)n2—, or substituted —(OCH2CH2)n2—, wherein:
n2 is 0-50; or
a group of the formula:
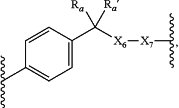 wherein:
Ra and Ra′ are each independently hydrogen, alkyl(C≤12), or substituted alkyl(C≤12);
X6 is O or —NR26R26′—, wherein:
R26 and R26′ are each independently alkyl(C≤12) or substituted alkyl(C≤12);
X7 is a covalent bond, O, S, —NH—, —(OCH2CH2)n3—, or substituted —(OCH2CH2)n3—, wherein:
n3 is 0-50; or
a group of the formula:
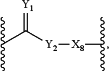 wherein:
Y is O or S;
Y2 is O, S, —NH—, or —NR27—, wherein:
R27 is alkyl(C≤12) or substituted alkyl(C≤12); and
X8 is a covalent bond, O, S, —NH—, —(OCH2CH2)n3—, or substituted —(OCH2CH2)n3—;
or a pharmaceutically acceptable salt thereof.
|