| CPC A61N 1/3956 (2013.01) [A61N 1/3943 (2013.01); A61N 1/3968 (2013.01)] | 15 Claims |
|
1. A subcutaneous implantable active medical device comprising:
a housing;
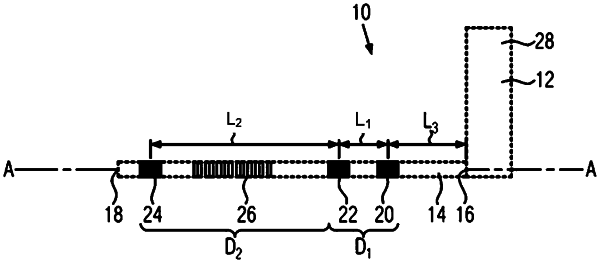
a subcutaneous implantable lead connected to the housing, the subcutaneous implantable lead comprising a plurality of sensing electrodes forming at least two dipoles on the subcutaneous implantable lead including a first dipole and a second dipole, from which at least two electrical signals are collected concurrently; and
a controller configured to:
perform an initial analysis that includes determining a two-dimensional curve parameterized as a function of time and representative of cardiac activity of a patient by plotting second electrical signals collected via the second dipole as a function of first electrical signals collected via the first dipole, and determining a tangent vector at a plurality of points of said two-dimensional curve;
utilize the two-dimensional curve to determine whether the collected signals include undesirable noise including identifying the undesirable noise in the collected signals as a function of a change of sign of at least one of coordinates of the tangent vector between each pair of successive points of the plurality of points of said two-dimensional curve;
in response to determining that the collected signals include the undesirable noise, perform the initial analysis again to determine an updated two-dimensional curve without the identified undesirable noise;
utilize the updated two-dimensional curve to confirm a presence of a tachyarrhythmia episode on a basis of a criterion of similarity between said updated two-dimensional curve and a reference two-dimensional curve that is representative of a normal sinus rhythm; and
subsequent to confirming the presence of the tachyarrhythmia episode, trigger a defibrillation operation.
|