| CPC A61M 5/1723 (2013.01) [A61B 5/4519 (2013.01); A61B 5/4869 (2013.01); A61B 6/032 (2013.01); A61B 6/463 (2013.01); A61B 6/5205 (2013.01); A61B 6/5217 (2013.01); G06T 7/0014 (2013.01); G16H 20/60 (2018.01); G16H 30/20 (2018.01); G16H 30/40 (2018.01); G16H 50/20 (2018.01); A61M 5/14228 (2013.01); A61M 5/14546 (2013.01); A61M 2205/3584 (2013.01); G06T 2207/10081 (2013.01); G06T 2207/20072 (2013.01); G06T 2207/30008 (2013.01)] | 20 Claims |
|
1. A parenteral nutritional diagnostic system comprising:
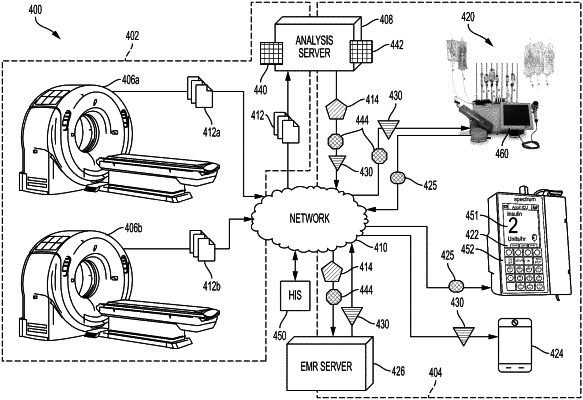
a soft tissue analysis server communicatively coupled to a computed tomography (“CT”) imaging device and configured to:
receive a two-dimensional image of a mid-section of a patient specifying radiodensity data of tissue,
create a radiodensity distribution plot of the tissue,
locate a soft tissue peak within the radiodensity distribution plot that corresponds to a local peak in a range of −50 Hounsfield Units (“HU”) and 80 HU, and
transmit an indication of the soft tissue peak; and
a pharmacy preparation system communicatively coupled to the soft tissue analysis server and configured to:
receive the indication of the soft tissue peak,
determine a base dosing regimen as a total volume of a nutrition solution to be infused per day based on at least a gender, a height, and an ideal body weight of the patient,
adjust the base dosing regimen based on the soft tissue peak,
determine administration parameters for a parenteral nutrition pump based on the adjusted base dosing regimen, and
transmit an administration message to the parenteral nutrition pump to cause a nutritional therapy to be administered to the patient according to the administration parameters.
|