| CPC A61K 9/2013 (2013.01) [A61K 9/28 (2013.01); A61K 31/195 (2013.01)] | 9 Claims |
|
1. A tablet comprising:
[(1R,5S,6S)-6-(aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetic acid monobenzenesulfonate of formula (I):
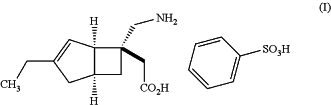 D-mannitol;
carmellose calcium;
citric acid anhydride or citric acid hydrate, wherein the citric acid anhydride or citric acid hydrate is present at 1.125 to 2.9% by weight of the total weight of the uncoated tablet; and
α-tocopherol, wherein the α-tocopherol is present at 0.005 to 0.5% by weight of the total weight of the uncoated tablet.
|