| CPC A61K 9/1272 (2013.01) [A61K 31/7105 (2013.01); A61K 31/711 (2013.01); A61K 39/12 (2013.01)] | 17 Claims |
|
1. A method of inducing an immune response in a subject comprising administering to the subject a pharmaceutical composition, the pharmaceutical composition comprising
a lipid-NA nanoparticle comprising lipids and a nucleic acid (NA), the lipid-NA particle encapsulating the nucleic acid;
wherein the lipids comprise a phospholipid, a cholesterol, a PEG-lipid, and a lipid of formula I
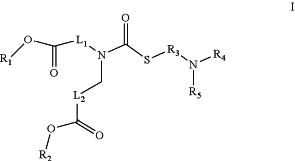 wherein
R1 and R2 are the same or different, each a linear or branched alkyl or alkenyl consisting of 3 to 31 carbons,
R3 is a linear or branched alkylene consisting of 1 to 6 carbons,
R4 and R5 are the same or different, each a hydrogen or a linear or branched alkyl consisting of 1 to 6 carbons;
L1 is a linear alkylene of 1 to 20 carbons or a linear alkenylene of 2 to 20 carbons,
L2 is a linear alkylene of 1 to 20 carbons, a linear alkenylene of 2 to 20 carbons, or L2 is a bond; and
wherein the nucleic acid encodes an immunogenic peptide and the nucleic acid has a nucleotide (nt) length of 200 to 25,000 nt.
|