| CPC A61K 31/713 (2013.01) [A61K 47/549 (2017.08); A61P 31/20 (2018.01)] | 26 Claims |
|
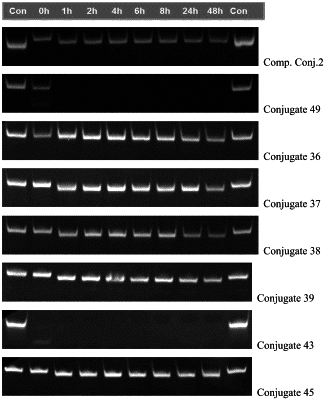
1. A siRNA conjugate having a structure as shown by Formula (1):
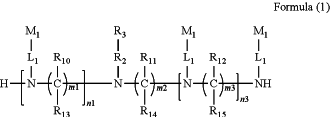 wherein,
n1 is an integer of 1-2 and n3 is an integer of 0-1, and n1+n3=2-3;
each of m1, m2, and m3 is independently an integer of 2-10;
each of R10, R11, R12, R13, R14 and R15 is independently H or selected from the group consisting of C1-C10 alkyl, C1-C10 haloalkyl and C1-C10 alkoxy;
R3 is a group having a structure as shown by Formula A59:
 wherein E1 is OH, SH or BH2; and Nu is siRNA;
each nucleotide in the siRNA is independently a modified or unmodified nucleotide; the siRNA comprises a sense strand and an antisense strand, wherein the sense strand comprises a nucleotide sequence 1, and the antisense strand comprises a nucleotide sequence 2; the nucleotide sequence 1 and the nucleotide sequence 2 are at least partly reverse complementary to form a double-stranded region; the nucleotide sequence 1 has the same length and no more than 3 nucleotides different from the nucleotide sequence shown in SEQ ID NO:155; and the nucleotide sequence 2 has the same length and no more than 3 nucleotides different from the nucleotide sequence shown in SEQ ID NO:156:
| ||||||||||||
wherein,
Z is A; Z′ is U;
the nucleotide sequence 1 comprises nucleotide ZA at the corresponding site to Z;
the nucleotide sequence 2 comprises nucleotide Z′B at the corresponding site to Z′; the nucleotide Z′B is the first nucleotide from 5′ terminal of the antisense strand;
R2 is a linear alkylene of 1 to 20 carbon atoms in length, wherein one or more carbon atoms are optionally replaced with any one or more of the group consisting of: C(O), NH, O, S, CH═N, S(O)2, C2-C10 alkeylene, C2-C10 alkynylene, C6-C10 arylene, C3-C18 heterocyclylene, and C5-C10 heteroarylene, and wherein R2 is optionally substituted by any one or more of the group consisting of: C1-C10 alkyl, C6-C10 aryl, C5-C10 heteroaryl, C1-C10 haloalkyl, —OC1-C10 alkyl, —OC1-C10 alkylphenyl, —C1-C10 alkyl-OH, —OC1-C10 haloalkyl, —SC1-C10 alkyl, —SC1-C10 alkylphenyl, —C1-C10 alkyl-SH, —SC1-C10 haloalkyl, halo, —OH, —SH, —NH2, —C1-C10 alkyl-NH2, —N(C1-C10 alkyl)(C1-C10 alkyl), —NH(C1-C10 alkyl), cyano, nitro, —CO2H, —C(O)O(C1-C10 alkyl), —CON(C1-C10 alkyl)(C1-C10 alkyl), —CONH(C1-C10 alkyl), —CONH2, —NHC(O)(C1-C10 alkyl), —NHC(O)(phenyl), —N(C1-C10 alkyl)C(O)(C1-C10 alkyl), —N(C1-C10 alkyl)C(O)(phenyl), —C(O)C1-C10 alkyl, —C(O)C1-C10 alkylphenyl, —C(O)C1-C10 haloalkyl, —OC(O)C1-C10 alkyl, —SO2(C1-C10 alkyl), —SO2(phenyl), —SO2(C1-C10 haloalkyl), —SO2NH2, —SO2NH(C1-C10 alkyl), —SO2NH(phenyl), —NHSO2(C1-C10 alkyl), —NHSO2(phenyl), and —NHSO2(C1-C10 haloalkyl);
each L1 is independently a linear alkylene of 1 to 70 carbon atoms in length, wherein one or more carbon atoms are optionally replaced with any one or more of the group consisting of: C(O), NH, O, S, CH═N, S(O)2, C2-C10 alkeylene, C2-C10 alkynylene, C6-C10 arylene, C3-C18 heterocyclylene, and C5-C10 heteroarylene, and wherein L1 is optionally substituted by any one or more of the group consisting of: C1-C10 alkyl, C6-C10 aryl, C5-C10 heteroaryl, C1-C10 haloalkyl, —OC1-C10 alkyl, —OC1-C10 alkylphenyl, —C1-C10 alkyl-OH, —OC1-C10 haloalkyl, —SC1-C10 alkyl, —SC1-C10 alkylphenyl, —C1-C10 alkyl-SH, —SC1-C10 haloalkyl, halo, —OH, —SH, —NH2, —C1-C10 alkyl-NH2, —N(C1-C10 alkyl)(C1-C10 alkyl), —NH(C1-C10 alkyl), cyano, nitro, —CO2H, —C(O)O(C1-C10 alkyl), —CON(C1-C10 alkyl)(C1-C10 alkyl), —CONH(C1-C10 alkyl), —CONH2, —NHC(O)(C1-C10 alkyl), —NHC(O)(phenyl), —N(C1-C10 alkyl)C(O)(C1-C10 alkyl), —N(C1-C10 alkyl)C(O)(phenyl), —C(O)C1-C10 alkyl, —C(O)C1-C10 alkylphenyl, —C(O)C1-C10 haloalkyl, —OC(O)C1-C10 alkyl, —SO2(C1-C10 alkyl), —SO2(phenyl), —SO2(C1-C10 haloalkyl), —SO2NH2, —SO2NH(C1-C10 alkyl), —SO2NH(phenyl), —NHSO2(C1-C10 alkyl), —NHSO2(phenyl), and —NHSO2(C1-C10 haloalkyl);
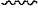 represents a site where a group is attached to the rest of the molecule; represents a site where a group is attached to the rest of the molecule;M1 represents a targeting group.
|