| CPC A61K 31/506 (2013.01) [A61K 31/137 (2013.01); A61K 31/277 (2013.01); A61K 31/4164 (2013.01); A61K 31/444 (2013.01); A61K 31/445 (2013.01); A61K 31/4545 (2013.01); A61K 31/505 (2013.01); A61K 45/06 (2013.01); C07C 217/58 (2013.01); C07C 255/61 (2013.01); C07D 211/60 (2013.01); C07D 233/26 (2013.01); C07D 239/06 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01)] | 26 Claims |
|
1. A compound of formula (I):
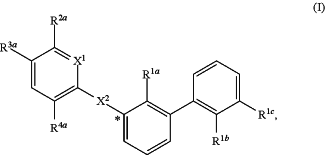 wherein:
X1 is selected from the group consisting of CH and N;
X2 is selected from the group consisting —OCH2—**, —CH2O—**, —C(═O)NH—**, and —NHC(═O)—**, wherein the bond marked with ** is to the phenyl ring carbon marked with *;
R1a is selected from the group consisting of H, C1-C3 alkyl, C1-C3 alkoxy, cyano, halogen, and C1-C3 haloalkyl;
R1b is selected from the group consisting of H, C1-C3 alkyl, C1-C3 alkoxy, cyano, halogen, and C1-C3 haloalkyl;
R1c is selected from the group consisting of C1-C6 alkyl, —OH, C1-C6 alkoxy optionally substituted with at least one selected from the group consisting of OH, C1-C6 alkoxy, phenyl, and optionally substituted heterocyclyl,
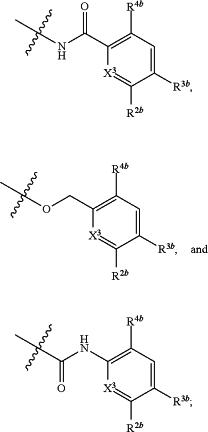 X3 is selected from the group consisting of CH and N;
R2a is selected from the group consisting of C1-C6 alkyl, C1-C6 alkoxy, —(CH2)1-3 (optionally substituted phenyl), —(CH2)1-3 (optionally substituted heteroaryl), —O(CH2)1-3 (optionally substituted phenyl), —O(CH2)1-3 (optionally substituted heteroaryl), —(CH2)1-3C(═O)OR, —(CH2)1-3C(═O)NRIRI, —O(CH2)1-3C(═O)ORI, and —O(CH2)1-3C(═O)NRIRI,
wherein each occurrence of R is independently H or C1-C6 alkyl optionally substituted with halogen, —OH, C1-C6 alkoxy, —NH2, —NH(C1-C6 alkyl), or —N(C1-C6 alkyl)(C1-C6 alkyl),
or two RI can combine with the N atom to which they are bound to form a 3-8 membered optionally substituted heterocyclyl;
R2b is selected from the group consisting of C1-C6 alkyl, C1-C6 alkoxy, —(CH2)1-3 (optionally substituted phenyl), —(CH2)1-3 (optionally substituted heteroaryl), —O(CH2)1-3 (optionally substituted phenyl), —O(CH2)1-3 (optionally substituted heteroaryl), —(CH2)1-3C(═O)ORII, —(CH2)1-3C(═O)NRIIRII, —O(CH2)1-3C(═O)ORII, and —O(CH2)1-3C(═O)NRIIRII,
wherein each occurrence of RII is independently H or C1-C6 alkyl optionally substituted with halogen, —OH, C1-C6 alkoxy, —NH2, —NH(C1-C6 alkyl), or —N(C1-C6 alkyl)(C1-C6 alkyl),
or two RII can combine with the N atom to which they are bound to form a 3-8 membered optionally substituted heterocyclyl,
R3a is selected from the group consisting of —CHO, —C(O)ORIII, —C(═O)NRIIIRIII, —C(═NR5)NRIIIRIII, optionally substituted heterocyclyl, —(CH2)1-3 (optionally substituted heterocyclyl), optionally substituted C1-C6 alkoxy, optionally substituted C1-C6 aminoalkyl, and optionally substituted C1-C6 hydroxyalkyl,
wherein each occurrence of RIII is independently H or C1-C6 alkyl optionally substituted with halogen, —OH, C1-C6 alkoxy, —NH2, —NH(C1-C6 alkyl), or —N(C1-C6 alkyl)(C1-C6 alkyl),
wherein each occurrence of R5 is independently H or C1-C6 alkyl optionally substituted with halogen, —OH, C1-C6 alkoxy, —NH2, —NH(C1-C6 alkyl), or —N(C1-C6 alkyl)(C1-C6 alkyl),
or two RIII can combine with the N atom to which they are bound to form a 3-8 membered optionally substituted heterocyclyl,
or, if R3a is —C(═NR5)NRIIIRIII, then R5 and one RIII can combine to form a 4-8 membered optionally substituted heterocyclyl;
R3b is selected from the group consisting of —CHO, —C(O)ORIV, —C(═O)NRIVRIVC(═NR5)NRIVRIV, optionally substituted heterocyclyl, —(CH2)1-3 (optionally substituted heterocyclyl), optionally substituted C1-C6 alkoxy, optionally substituted C1-C6 aminoalkyl, and optionally substituted C1-C6 hydroxyalkyl,
wherein each occurrence of RIV is independently H or C1-C6 alkyl optionally substituted with halogen, —OH, C1-C6 alkoxy, —NH2, —NH(C1-C6 alkyl), or —N(C1-C6 alkyl)(C1-C6 alkyl),
wherein each occurrence of R5 is independently H or C1-C6 alkyl optionally substituted with halogen, —OH, C1-C6 alkoxy, —NH2, —NH(C1-C6 alkyl), or —N(C1-C6 alkyl)(C1-C6 alkyl),
or two RIV can combine with the N atom to which they are bound to form a 3-8 membered optionally substituted heterocyclyl,
or, if R3b is —C(═NR5)NRIVRIV, then R5 and one RIV can combine to form a 4-8 membered optionally substituted heterocyclyl;
R4a is selected from the group consisting of H, halogen, cyano, and C1-C3 alkyl; and
R4b is selected from the group consisting of H, halogen, cyano, and C1-C3 alkyl;
or a salt, solvate, geometric isomer, stereoisomer, tautomer, and any mixtures thereof.
|