| CPC A61K 31/4155 (2013.01) [A61K 31/343 (2013.01); A61K 31/351 (2013.01); A61K 31/357 (2013.01); A61K 31/4025 (2013.01); A61K 31/403 (2013.01); A61K 31/41 (2013.01); A61K 31/426 (2013.01); A61K 31/443 (2013.01); A61K 31/4439 (2013.01); A61K 31/4525 (2013.01); A61K 31/506 (2013.01); A61P 35/00 (2018.01); C07D 307/81 (2013.01); C07D 405/04 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 407/04 (2013.01); C07D 407/12 (2013.01); C07D 417/04 (2013.01)] | 33 Claims |
|
1. A compound of formula (Ia), or a pharmaceutically acceptable salt thereof,
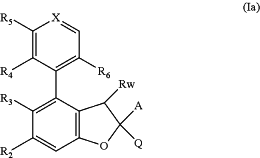 wherein
X is selected from CH and N;
A is selected from
(i) phenyl, wherein the phenyl is optionally substituted with halo; or haloC1-C3alkoxy;
(ii) a 5- or 6-membered aromatic heterocyclic ring comprising at least one heteroatom selected from N, O, and S, wherein the aromatic heterocyclic ring is optionally substituted with hydroxy; C1-C3alkoxy; or oxo; and
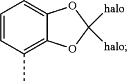
(iii) a halobenzodioxole moiety of formula
 Rw is selected from (i) hydrogen; (ii) hydroxy; (iii) C1-C3alkoxy; (iv) hydroxy-C1-C3alkyl; (v) C1-C3alkyl; and (vi) C1-C3alkoxy-C1-C3alkyl;
Q is selected from (i) —C(R7)2-N(R8)—R1; (ii) 9- or 10-membered partially saturated heteroaryl comprising at least one N heteroatom; and (iii) 4-, 5- or 6-membered saturated heterocyclic ring comprising at least one heteroatom or heteroatom group selected from N, O, S, —S(═O) and —S(═O)2, with the proviso that at least one N heteroatom is present, wherein the heterocyclic ring is unsubstituted or substituted with one or more substituents independently selected from hydroxy, C1-C3alkyl, C1-C3alkoxy, halo and C1-C3alkylene forming a bridge between two ring atoms of the saturated heterocyclic ring, thus forming a bridged bicyclic structure;
R1 is selected from (i) hydrogen; (ii) C1-C6alkyl which is optionally deuterated; and (iii) (CH2)0-2R1a;
R1a is selected from (i) hydroxyC1-C4alkyl; (ii) C1-C3alkoxy; (iii) a 5- or 6-membered saturated heterocyclic ring comprising at least one heteroatom selected from N and O, wherein the saturated heterocyclic ring is optionally substituted with one or more substituents independently selected from C1-C3alkyl; (CH2)0-1C(O)di(C1-C3alkyl)amino; SO2C1-C3alkyl; C(O)C1-C3alkyl; or oxo; and (iv) C3-C6cycloalkyl optionally substituted with one or more substituents independently selected from hydroxy; hydroxyC1-C4alkyl; C1-C6alkoxy; C(O)OC1-C3alkyl; CO2H; SO2C1-C3alkyl; haloC1-C3alkyl; NHR1b; (CH2)0-1C(O)NR1cR1d; C1-C6alkyl; haloC1-C3alkoxy-C1-C3alkyl; halo; a 5- or 6-membered aromatic heterocyclic ring comprising at least one heteroatom selected from N, O, and S; and two R1c groups,
wherein the two R1c attached at the same carbon atom form together with the carbon atom to which they are attached a 5-membered saturated heterocyclic ring comprising at least one heteroatom selected from N and O, or a C3-C6cycloalkyl, wherein the saturated heterocyclic ring or cycloalkyl are optionally substituted with hydroxy or oxo;
R1b is selected from (i) C(O)C1-C3alkyl; and (ii) SO2C1-C3alkyl;
R1c and R1d are each independently selected from (i) hydrogen; (ii) C1-C3alkyl; and (iii) hydroxyC1-C4alkyl;
R2 is selected from (i) hydrogen; and (ii) halo;
R3 is selected from (i) halo; (ii) haloC1-C3alkyl; and (iii) cyano;
R4 is selected from (i) hydrogen; (ii) halo; and (iii) C1-C3alkyl;
R5 is selected from (i) hydrogen; (ii) C1-C6alkoxy optionally substituted with C3-C6cycloalkyl; CO2H; SO2C1-C3alkyl; a 5- or 6-membered aromatic heterocyclic ring comprising at least one heteroatom selected from N, O, and S; or a 5- or 6-membered saturated heterocyclic ring comprising at least one heteroatom selected from N and O, wherein the ring is optionally substituted with C(O)C1-C3alkyl; (iii) halo; (iv) hydroxyC1-C6alkoxy, wherein the alkoxy is optionally deuterated; (v) haloC1-C6alkoxy optionally substituted with hydroxy; (vi) S-haloC1-C3alkyl optionally substituted with hydroxy; (vii) C1-C3alkoxyC1-C3alkoxy; (viii) NR5aR5b; (ix) C1-C3alkyl; (x) a 5- or 6-membered aromatic heterocyclic ring comprising at least one heteroatom selected from N, O, and S; and (xi) hydroxy;
R5a and R5b are each independently selected from (i) hydrogen; and (ii) C1-C3alkyl; or
R5a and R5b together with the nitrogen atom to which they are attached form a 5- or 6-membered saturated heterocyclic ring, wherein the saturated heterocyclic ring optionally in addition carries a hydroxy group;
R6 is selected from (i) hydrogen; (ii) cyano; (iii) C(O)NR6a; (iv) NHR6b; and (v) C1-C3alkoxy substituted with NH2 or hydroxy;
R6a is selected from (i) hydrogen; (ii) C1-C3alkyl; (iii) C3-C6cycloalkyl; and (iv) a 5- or 6-membered aromatic heterocyclic ring comprising at least one heteroatom selected from N, O, and S, wherein the aromatic heterocyclic ring is optionally substituted with C1-C3alkyl;
R6b is C1-C3alkyl substituted with NH2 or hydroxy;
R7 is each independently selected from hydrogen and C1-C3alkyl; and
R8 is hydrogen or C1-C3-alkyl.
|