| CPC A61K 31/407 (2013.01) [A61K 9/0073 (2013.01); A61P 11/00 (2018.01)] | 8 Claims |
|
1. A method of treating idiopathic pulmonary fibrosis (IPF) in a mammalian subject, comprising administering to the mammalian subject, an effective amount of a compound of Formula I
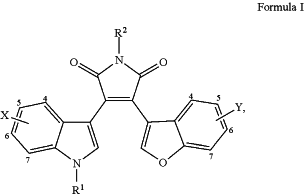 or a pharmaceutically acceptable salt or solvate thereof, wherein:
X is independently —H, halo, 5,6-methylenedioxy, —CN, —OMe, —OH, —OBn, —CF3, —CH2OH, —CH2OMe, —CH2CH2CO2H, or —CH2CH2CO2Et;
R1 is —H, lower alkyl, lower alkyl substituted with a hydroxy, or lower alkyl substituted with —NH2;
R2 is —H, lower alkyl, lower alkyl substituted with a hydroxy, or lower alkyl substituted with —NH2; and
Y is independently —H, halo, —OMe, —OH, —OBn, —CF3, —CH2OH, —CH2OMe, —NO2, —CN, or —C═CH2.
|