| CPC A61F 9/00781 (2013.01) [A61F 2210/0014 (2013.01); A61F 2250/0067 (2013.01)] | 33 Claims |
|
1. A device for maintaining patency of a channel of an uveolymphatic region or a Schlemm's canal in a patient's eye, said device comprising:
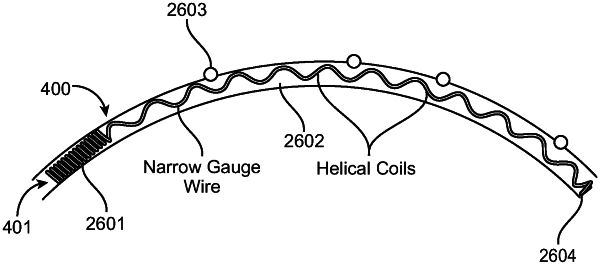
an expansion member consisting of a single elongated element for radial expansion of the channel when inserted into the channel, the single elongate element consisting essentially of a single pre-shaped metal or polymeric filament that is biased to assume a curved configuration through its entire length to match a radius of curvature of the channel,
wherein the single elongated element is a shape memory alloy wire having a diameter in a range from 0.001 mm to 1 mm and formed into a cylindrical helix having a central region with a pitch between successive turns in a range from 0.001 mm to 10 mm and diameter in a range from 0.001 mm to 10 mm when unconstrained;
wherein the expansion member in its curved configuration has (i) sufficient radial strength to withstand compressive stresses exerted by the channel and (ii) sufficient void space in its structure to minimize blockage of the blocking of collector channels in the channel, when the expansion member is implanted in the channel.
|