| CPC H10K 85/342 (2023.02) [C07F 15/0033 (2013.01); C09K 11/06 (2013.01); C09K 2211/1007 (2013.01); C09K 2211/1029 (2013.01); C09K 2211/1033 (2013.01); C09K 2211/1044 (2013.01); C09K 2211/185 (2013.01); H10K 50/11 (2023.02); H10K 50/12 (2023.02); H10K 50/81 (2023.02); H10K 50/82 (2023.02); H10K 85/6572 (2023.02); H10K 85/6576 (2023.02); H10K 2101/10 (2023.02)] | 20 Claims |
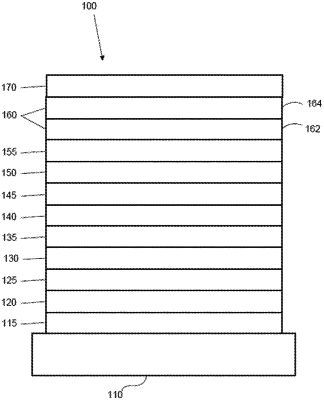
|
1. A composition comprising a first compound;
wherein the first compound is capable of functioning as a phosphorescent emitter in an organic light emitting device at room temperature;
wherein the first compound has at least one aromatic ring and at least one substituent R;
wherein each of the at least one R is independently selected from the group consisting of partially fluorinated alkyl, partially fluorinated cycloalkyl, and combinations thereof,
wherein each of the at least one R is directly bonded to one of the aromatic rings;
wherein in each of the at least one R, a C having an F attached thereto is separated by at least one carbon atom from the aromatic ring;
wherein the first compound has the formula of M(L1)x(L2)y(L3)z;
wherein M is selected from the group consisting of Ir, Rh, Re, Ru, Os, Pt, Au, and Cu;
wherein x is 1, 2, or 3;
wherein y is 0, 1, or 2;
wherein z is 0, 1, or 2;
wherein x+y+z is the oxidation state of the metal M;
wherein when L1, L2, and L3 are each present, at least one of L1, L2, and L3 is different from the others;
wherein L1, L2, and L3 are each independently selected from the group consisting of:
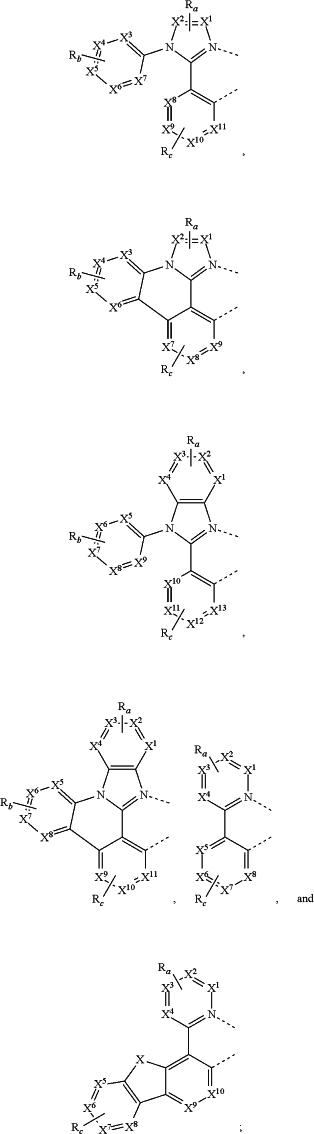 wherein each X1 to X13 are independently selected from the group consisting of carbon and nitrogen;
wherein X is selected from the group consisting of BR′, NR′, PR′, O, S, Se, C═O, S═O, SO2, CR′R″, SiR′R″, and GeR′R″;
wherein R′ and R″ are optionally fused or joined to form a ring;
wherein each Ra, Rb, Rc, and Rd may represent from a mono substitution to a maximum possible number of substitutions, or no substitution;
wherein each of R′, R″, Ra, Rb, Rc, and Rd is independently selected from the group consisting of hydrogen, deuterium, halide, alkyl, cycloalkyl, heteroalkyl, arylalkyl, alkoxy, aryloxy, amino, silyl, alkenyl, cycloalkenyl, heteroalkenyl, alkynyl, aryl, heteroaryl, acyl, carbonyl, carboxylic acids, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl, phosphino, and combinations thereof; wherein any two adjacent substituents of Ra, Rb, Rc, and Rd are optionally fused or joined to form a ring or form a multidentate ligand; and
wherein at least one Rc comprises at least one R.
|