| CPC C12N 15/907 (2013.01) [C12N 9/22 (2013.01); C12N 15/11 (2013.01); C12N 15/86 (2013.01); C12N 2750/14143 (2013.01)] | 30 Claims |
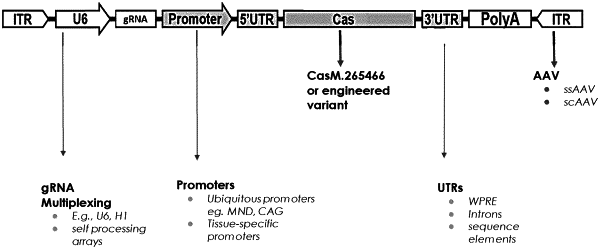
|
1. A composition comprising:
a) an engineered polypeptide or a nucleic acid encoding the engineered polypeptide, wherein the engineered polypeptide comprises an amino acid sequence that is at least 95% identical to SEQ ID NO: 1, and wherein the engineered polypeptide comprises at least one amino acid substitution relative to SEQ ID NO: 1; and
b) a guide nucleic acid comprising a first sequence and a second sequence,
wherein the engineered polypeptide is capable of binding the first sequence, and wherein the first sequence and the second sequence are heterologous, and
wherein the at least one amino acid substitution is selected from K58W, I80R, T84R, K105R, N193K, C202R, S209F, G210R, A218R, A218K, D220R, E225R, E225K, D237A, C246R, N286K, M298L, A306K, Y315M, E335A, E335Q, Q360R, D418A, and D418N.
|