| CPC C07D 471/04 (2013.01) [A61P 35/00 (2018.01); C07D 403/12 (2013.01); C07D 405/14 (2013.01); C07D 471/14 (2013.01); C07D 519/00 (2013.01)] | 23 Claims |
|
1. A method for inhibiting Bcl-2 proteins in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound of Formula (II):
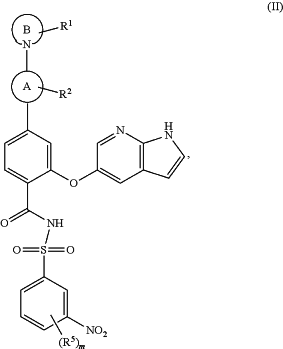 or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof,
wherein:
Ring A is 1,4-phenylene; or
Ring A is a 4-membered/4-membered, 3-membered/5-membered, 4-membered/5-membered, 4-membered/6-membered, 5-membered/5-membered, 5-membered/6-membered, or 6-membered/6-membered mono-spiro heterocyclyl comprising one or two nitrogen or oxygen atoms as ring members, each of which is optionally substituted with 1 to 4 substituents R2;
each R2 is independently selected from hydrogen, halogen, and —C1-8 alkyl optionally substituted with halogen;
Ring B is a monocyclic 4- to 9-membered heterocyclyl comprising one nitrogen atom as a ring member or a monocyclic 4- to 9-membered heterocyclyl comprising one nitrogen atom and one additional heteroatom selected from NH, O, S, S(O), and SO2 as ring members, wherein Ring B is N-linked to Ring A;
R1 is hydrogen, halogen, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, heteroaryl, oxo, —CN, —NO2, —OR1a, —SO2R1a, —COR1a, —CO2R1a, —CONR1aR1b, —C(═NR1a)NR1bR1c, —NR1aR1b, —NR1aCOR1b, —NR1aCONR1bR1c, —NR1aCO2R1b, —NR1aSONR1bR1c, —NR1aSO2NR1bR1c, or —NR1aSO2R1b, wherein the —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with 1 to 4 substituents R1d;
R1a, R1b, and R1c are each independently hydrogen, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each of the —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with halogen, hydroxy, or —C1-8 alkoxy;
each R1d is independently halogen, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, heteroaryl, oxo, —CN, —NO2, —ORBa, —SO2RBa, —CORBa, —CO2RBa, —CONRBaRBb, —C(═NRBa)NRBbRBbc, —NRBaRBb, —NRBaCORBb, —NRBaCONRBbRBc, —NRBaCO2RBb, —NRBaSONRBbRBc, —NRBaSO2NRBbRBc, or —NRBaSO2RBb, wherein the —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl are each independently optionally substituted with 1 to 4 substituents RBd;
RBa, RBb, and RBc are each independently hydrogen, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each of the —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with halogen, hydroxy, —NH2, —N(C1-6 alkyl)2, —C1-8 alkoxy, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
each RBd is independently halogen, oxo, —CN, —NO2, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each of the —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with halogen, hydroxy, —C1-8 alkoxy, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
R5 is -L5-CyC;
L5 is a direct bond, —(CRaRb)t—, —(CRaRb)t-1—(CRc═CRd)—(CRaRb)v-1—, —(CRaRb)t-1—(C≡C)—(CRaRb)v-1—, —O—, —S—, —S(O)—, —SO2—, —C(O)—, C(O)—, —OC(O)—, —NRa—, —C(O)NRa—, —NRaC(O)—, —NRaC(O)O—, —NRaC(O)NRb—, —SO2NRa—, —NRaSO2—, —NRaS(O)2NRb—, —NRaS(O)NRb—, —C(O)NRaSO2—, —C(O)NRaSO—, or —C(═NRa)NRb—, wherein t and v are each independently an integer of 1 to 7, and one or two CRaRb moieties in —(CRaRb)t—, —(CRaRb)t-1—(CRc═CRd)—(CRaRb)v-1—, or —(CRaRb)t-1—(C≡C)—(CRaRb)v-1— are optionally replaced with one or more moieties selected from O, S, SO, SO2, C(O), and NRa;
CyC is cycloalkyl, heterocyclyl, aryl, or heteroaryl, each of which is optionally substituted with one or two substituents R5a;
each R5a is independently halogen, cyano, oxo, —NO2, —OR5b, —SR5b, —NR5bR5c, —COR5b, —SO2R5b, —C(═O)OR5b, —C(═O)NR5bR5c, —C(═NR5b)NR5cR5d, —N(R5b)C(═O)R5c, —N(R5b)C(═O)OR5c, —N(R5b)C(O)NR5cR5d, —N(R5b)S(O)NR5cR5d, —N(R5b)S(O)2NR5cR5d, —NR5bSO2R5c, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each of the —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or two substituents R5e;
R5b, R5c, and R5d are each independently hydrogen, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each of the —C1-8 alkyl, —C2-8 alkenyl, C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or two substituents R5e;
each R5e is independently halogen, cyano, oxo, —NO2, —OR5f, —SR5f, —NR5fR5g, —COR5f, —SO2R5f, —C(═O)OR5f, —C(═O)NR5fR5g, —C(═NR5f)NR5gR5h, —N(R5f)C(═O)R5g, —N(R5f)C(═O)OR5g, —N(R5f)C(O)NR5gR5h, —N(R5f)S(O)NR5gR5h, —N(R5f)S(O)2NR5gR5h, —NR5fSO2R5g, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
R5f, R5g, and R5h are each independently hydrogen, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
or two adjacent R5 substituents on the phenyl ring together with the phenyl ring form a benzo ring, wherein the ring is optionally substituted with halogen, oxo, cyano, —NO2, —OR5i, —SR5i, —NR5iR5j, —COR5i, —SO2R5i, —C(═O)OR5i, —C(═O)NR5iR5j, —C(═NR5i)NR5jR5k, —N(R5i)C(═O)R5j, —N(R5i)C(═O)OR5j, —N(R5i)C(O)NR5jR5k, —N(R5i)S(O)NR5jR5k, —N(R5i)S(O)2NR5jR5k, —NR5iSO2R5k, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
R5i, R5j, and R5k are each independently hydrogen, —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each of the —C1-8 alkyl, —C2-8 alkenyl, —C2-8 alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with halogen, hydroxy, or —C1-8 alkoxy;
m is an integer of 1-4;
Ra, Rb, Rc, and Rd are each independently hydrogen, —C1-8alkyl, —C2-8alkenyl, —C2-8alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein said —C1-8alkyl, —C2-8alkenyl, —C2-8alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl are each independently optionally substituted with —CN, halogen, —NO2, —NReRf, oxo, —ORe, or —SRe; and
Re and Rf are each independently hydrogen, C1-8alkyl, C1-8alkoxy-C1-8alkyl-, C2-8alkenyl, C2-8alkynyl, cycloalkyl, aryl, heterocyclyl, or heteroaryl.
|