| CPC C07D 471/04 (2013.01) [A61K 45/06 (2013.01); C07B 2200/05 (2013.01)] | 22 Claims |
|
1. A pharmaceutical composition comprising a compound having structural formula I:
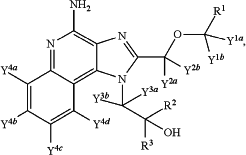 or a pharmaceutically acceptable salt thereof, wherein:
each of R1, R2 and R3 is independently selected from CH3 and CD3;
each of Y1a, Y1b, Y2a, Y2b, Y3a, Y3b, Y4a, Y4b, Y4c, and Y4d is independently selected from hydrogen and deuterium;
each position that is designated as containing deuterium has at least 50.1% deuterium incorporation at that position and at least R1 or both R2 and R3 are CD3, and
Y1a and Y1b are the same;
Y2a and Y2b are the same;
Y3a and Y3b are the same; and
Y4a, Y4b, Y4c, and Y4d are the same.
|