| CPC C07D 405/06 (2013.01) [C07B 2200/13 (2013.01)] | 22 Claims |
|
1. A process for preparing a 1-methoxyisoindoline of formula (1°):
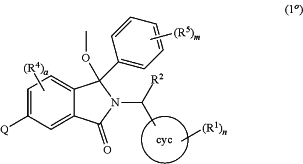 or a tautomer or a solvate or a salt thereof,
the process comprising taking a compound of the formula (2°)
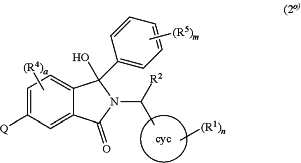 wherein cyc is phenyl or a heterocyclic group Het which is pyridinyl, pyrimidinyl, pyrazinyl or pyridazinyl, or an N-oxide thereof;
R1 is independently selected from hydroxy, halogen, nitro, nitrile, C1-4alkyl, haloC1-4alkyl, hydroxyC1-4alkyl, C2-6alkenyl, C1-4alkoxy, haloC1-4alkoxy, C2-4alkynyl, —O0,1—(CRxRy)v—CO2H, —(CRxRy)v—CO2C1-4alkyl, —(CRxRy)v—CON(C1-4alkyl)2, —P(═O)(Rx)2, —S(O)d—Rx, —S(O)d-heterocyclic group with 3 to 6 ring members and —S(O)d—N(R8)2, wherein when cyc is Het then R1 is attached to a carbon atom;
R2 is selected from hydrogen, C1-4alkyl, C2-6alkenyl, hydroxyC1-4alkyl, —(CRxRy)u—CO2H, —(CRxRy)u—CONRxRy, —(CRxRy)u—CO2R10 wherein R10 is selected from C1-7 alkyl, C1-7 alkeneyl, C1-7 haloalkyl, triC1-7 alkylsilyl-C1-7alkyl, C5-20 aryl and C5-20 aryl-C1-7 alkyl;
R4 and R5 are independently selected from halogen, nitrile, C1-4alkyl, haloC1-4alkyl, C1-4alkoxy and haloC1-4alkoxy;
Q is selected from —C(OH)R6R7, —C(═O)R7, halogen and —OTf;
R6 and R7 are independently selected from hydrogen, C1-6alkyl, haloC1-6alkyl, C2-6alkenyl, C2-6alkynyl, hydroxy, hydroxyC1-6alkyl, —COOC1-6alkyl, —(CH2)j—O—C1-6alkyl, —(CH2)j—O-(hydroxyC1-6alkyl), —C1-6alkyl-NRxRy, —(CRxRy)p—CONRxRy, —(CRxRy)p—NRxCORy, —(CRxRy)p—O—CH2—CONRxRy, heterocyclic group with 3 to 7 ring members, —CH2-heterocyclic group with 3 to 7 ring members, —CH2—O-heterocyclic group with 3 to 7 ring members, —CH2—NH-heterocyclic group with 3 to 7 ring members, —CH2—N(C1-6alkyl)-heterocyclic group with 3 to 7 ring members, —C(═O)NH-heterocyclic group with 3 to 7 ring members, C3-8cycloalkyl, —CH2—C3-8cycloalkyl, —CH2—O—C3-8cycloalkyl, and C3-8cycloalkenyl, wherein said cycloalkyl, cycloalkenyl or heterocyclic groups may be optionally substituted by one or more Rz groups, and wherein in each instance the heterocyclic group comprises one or more heteroatoms selected from N, O, S and oxidised forms thereof;
or, when Q is —C(OH)R6R7, the R6 and R7 groups, together with the carbon atom to which they are attached, can join to form a C3-6cycloalkyl or heterocyclyl group with 3 to 6 ring members, wherein the heterocyclic group comprises one or more heteroatoms selected from N, O, S and oxidised forms thereof, and wherein said C3-6cycloalkyl and heterocyclyl groups may be optionally substituted by one or more Rz groups;
R8 is selected from hydrogen, C1-6alkyl, haloC1-6alkyl, hydroxyC1-6alkyl, —(CH2)k—O—C1-6alkyl, —(CH2)k—O-(hydroxyC1-6alkyl), hydroxyC1-6alkoxy, —(CH2)k—CO2C1-6alkyl, —(CH2)k—CO2H, —C1-6alkyl-N(H)e(C1-4alkyl)2-e, —(CH2)j—C3-8cycloalkyl and —(CH2)j—C3-8cycloalkenyl;
Rx and Ry are independently selected from hydrogen, halogen, nitro, nitrile, C1-6alkyl, haloC1-6alkyl, C2-6alkenyl, C2-6alkynyl, hydroxy, hydroxyC1-6alkyl, C1-6alkoxy, —(CH2)k—O—C1-6alkyl, hydroxyC1-6alkoxy,
—COOC1-6alkyl, —N(H)e(C1-4alkyl)2-e, —C1-6alkyl-N(H)e(C1-4alkyl)2-e, —(CH2)k—C(═O)N(H)e(C1-4alkyl)2-e, C3-8cycloalkyl and C3-8cycloalkenyl;
or the Rx and Ry groups, together with the carbon or nitrogen atom to which they are attached, can join to form a C3-6cycloalkyl or saturated heterocyclyl group with 3 to 6 ring members which may be optionally fused to an aromatic heterocyclyl group of 3 to 5 ring members;
or when on a carbon atom the Rx and Ry groups can join together to form a ═CH2 group; Rz is independently selected from halogen, nitro, nitrile, C1-6alkyl, haloC1-6alkyl, C2-6alkenyl, C2-6alkynyl, ═O, hydroxy, hydroxyC1-6alkyl, C1-6alkoxy, —(CH2)—O—C1-6alkyl, hydroxyC1-6alkoxy, —C(═O)C1-6alkyl, C(═O)C1-6alkyl-OH, —C(═O)C1-6alkyl-N(H)e(C1-4alkyl)2-e, —C(═O)N(H)e(C1-4alkyl)2-e, —(CH2)r—CO2C1-6alkyl, —(CH2)r—CO2H, —N(H)e(C1-4alkyl)2-e, —C1-6alkyl-N(H)e(C1-4alkyl)2-e, heterocyclyl group with 3 to 6 ring members, heterocyclyl group with 3 to 6 ring members substituted by —C(═O)C1-4alkyl, heterocyclyl group with 3 to 6 ring members substituted by —C(═O)OC1-4alkyl, heterocyclyl group with 3 to 6 ring members substituted by —C(═O)N(H)e(C1-4alkyl)2-e, —C(═O)heterocyclyl group with 3 to 6 ring members, C3-8cycloalkyl and C3-8cycloalkenyl, wherein if R7 is pyridine then Rz is other than —NH2;
a, j, d, e, n, r and p are independently selected from 0, 1 and 2;
k and m are independently selected from 1 and 2;
u is selected from 0, 1, 2 and 3; and
v is selected from 0 and 1;
and reacting the compound of formula (2°) with a methylating agent in the presence of a base.
|