| CPC A61K 47/6889 (2017.08) [A61K 47/6803 (2017.08); A61K 47/6855 (2017.08); C07K 16/32 (2013.01); C07K 2317/94 (2013.01)] | 27 Claims |
|
1. A method of treating breast cancer in a subject, the method comprising:
administering to the subject a therapeutically effective amount of a pharmaceutical composition comprising a conjugate, wherein the administering is effective to treat breast cancer in the subject, and
wherein the conjugate is of formula (I):
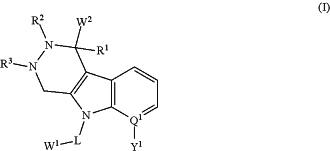 wherein
Q1 is C or N, wherein if Q1 is N, then Y1 is absent;
Y1 is selected from hydrogen, halogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, alkoxy, substituted alkoxy, amino, substituted amino, carboxyl, carboxyl ester, acyl, acyloxy, acyl amino, amino acyl, alkylamide, substituted alkylamide, sulfonyl, thioalkoxy, substituted thioalkoxy, aryl, substituted aryl, heteroaryl, substituted heteroaryl, cycloalkyl, substituted cycloalkyl, heterocyclyl, and substituted heterocyclyl;
R1 is selected from hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, cycloalkyl, substituted cycloalkyl, heterocyclyl, and substituted heterocyclyl;
R2 and R3 are each independently selected from hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, alkoxy, substituted alkoxy, amino, substituted amino, carboxyl, carboxyl ester, acyl, acyloxy, acyl amino, amino acyl, alkylamide, substituted alkylamide, sulfonyl, thioalkoxy, substituted thioalkoxy, aryl, substituted aryl, heteroaryl, substituted heteroaryl, cycloalkyl, substituted cycloalkyl, heterocyclyl, and substituted heterocyclyl, or R2 and R3 are optionally cyclically linked to form a 5 or 6-membered heterocyclyl;
L is a linker comprising -(T1-Z1)a-(T2-Z2)b-(T3-Z3)c-(T4-Z4)a, wherein a, b, c, and d are each independently 0 or 1, where the sum of a, b, c, and dis 1 to 4;
T1, T2, T3, and T4 are each independently selected from (C1-C12)alkyl, substituted (C1-C12)alkyl, (EDA)w, (PEG)n, (AA)p, —(CR13OH)h—, piperidin-4-amino (4AP), an acetal group, a hydrazine, and an ester, wherein EDA is an ethylene diamine moiety, PEG is a polyethylene glycol or a modified polyethylene glycol, and AA is an amino acid residue, wherein w is an integer from 1 to 20, n is an integer from 1 to 30, p is an integer from 1 to 20, and h is an integer from 1 to 12;
Z1, Z2, Z3, and Z4 are each independently selected from the group consisting of a covalent bond, —CO—, —NR15—, —NR15(CH2)q—, —NR15(C6H4)—, —CONR15—, —NR15CO—, —C(O)O—, —OC(O)—, —O—, —S—, —S(O)—, —SO2—, —SO2NR15—, —NR15SO2— and —P(O)OH—, wherein q is an integer from 1 to 6;
each R13 is independently selected from hydrogen, an alkyl, a substituted alkyl, an aryl, and a substituted aryl;
each R15 is independently selected from hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, carboxyl, carboxyl ester, acyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, cycloalkyl, substituted cycloalkyl, heterocyclyl, and substituted heterocyclyl;
W1 is a maytansinoid; and
W2 is an anti-HER2 antibody comprising a sulfatase motif comprising a formylglycine residue, wherein the formylglycine residue is coupled to the maytansinoid through the linker.
|