| CPC A61K 31/7076 (2013.01) [A61K 9/0048 (2013.01); A61K 47/38 (2013.01); A61P 27/02 (2018.01)] | 8 Claims |
|
1. A method of treating optic nerve disease in a subject comprising:
administering a pharmaceutical composition comprising a compound represented by Chemical Formula 1 or a pharmaceutically acceptable salt thereof as an active ingredient to the subject:
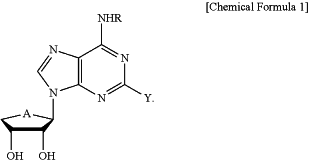 wherein
A is O or S,
R is
a) straight or branched C1 to C5 alkyl unsubstituted, or independently or optionally substituted with 1 or 2 or more C6 to C10 aryl,
b) benzyl unsubstituted, or independently or optionally substituted with 1 or 2 or more fluoro, chloro, bromo or straight or branched C1 to C4 alkoxy or
c) benzyl substituted with hydroxycarbonyl, and
Y is H or a halogen element,
wherein the optic nerve disease is selected from the group consisting of ischemic optic neuropathy, traumatic optic neuropathy, and compressive optic neuropathy.
|