| CPC A61B 17/12172 (2013.01) [A61B 17/0057 (2013.01); A61B 17/12113 (2013.01); A61B 17/12163 (2013.01); A61B 2017/00632 (2013.01); A61B 2017/00862 (2013.01); A61B 2017/00867 (2013.01); A61B 2017/12063 (2013.01); A61B 2090/3966 (2016.02)] | 20 Claims |
|
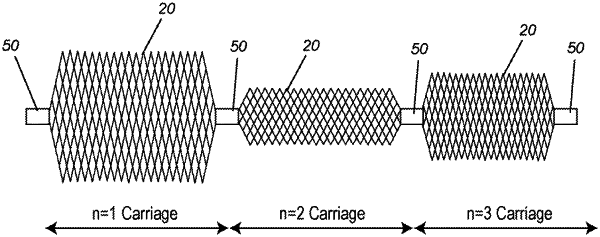
1. An occlusion device for implantation into a body lumen or aneurysm comprising:
a continuous mesh structure, wherein the continuous mesh structure comprises a plurality of axial mesh carriages configured end to end, wherein the continuous mesh structure is compressible; and
a plurality of markers encircling the continuous mesh structure at a plurality of pinch points in the continuous mesh structure,
wherein each end of each axial mesh carriage is (i) at a respective pinch point of the plurality of pinch points in the continuous mesh structure and (ii) encircled by a respective marker of the plurality of markers,
wherein the continuous mesh structure comprises a plurality of strands that each continuously extend across all of the plurality of axial mesh carriages, and
wherein each axial mesh carriage has a width (y) and length (x) that is different from the length and width of adjacent carriages of the plurality of axial mesh carriages.
|