| CPC H04W 4/80 (2018.02) [A61M 1/74 (2021.05); A61M 1/96 (2021.05); G16H 40/20 (2018.01); G16H 40/60 (2018.01); H04B 17/318 (2015.01); H04W 64/006 (2013.01); A61M 2205/18 (2013.01); A61M 2205/3561 (2013.01); A61M 2205/3584 (2013.01); A61M 2205/3592 (2013.01); A61M 2205/50 (2013.01); A61M 2205/502 (2013.01); A61M 2205/505 (2013.01); A61M 2205/52 (2013.01); A61M 2205/702 (2013.01); A61M 2205/8206 (2013.01); H04W 84/18 (2013.01)] | 14 Claims |
|
1. A network system for a hospital, the system comprising:
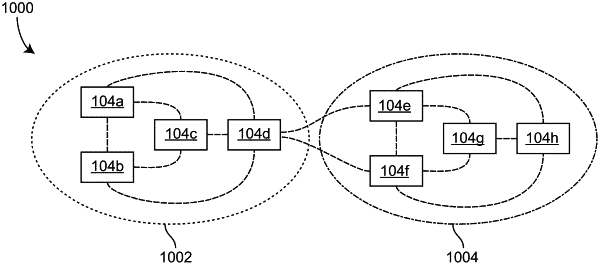
a plurality of negative pressure wound therapy devices forming a mesh network for the hospital, wherein the plurality of negative pressure wound therapy devices comprises a first neighborhood including a first set of negative pressure wound therapy devices configured to directly wirelessly communicate with each other to form the first neighborhood and a second neighborhood including a second set of negative pressure wound therapy devices configured to directly wirelessly communicate with each other to form the second neighborhood, at least one of the first neighborhood or the second neighborhood including a first negative pressure wound therapy device and a second negative pressure wound therapy device; and
an external network configured to communicatively couple with at least one of the plurality of negative pressure wound therapy devices;
wherein the first negative pressure wound therapy device comprises:
a wireless radio configured to wirelessly communicate with the second negative pressure wound therapy device;
a user interface configured to display to a user an operational status of a therapy operation of the first negative pressure wound therapy device, receive a command of an operational change from the user, and display to the user a wireless radio connection strength between the first negative pressure wound therapy device and the second negative pressure wound therapy device;
a controller configured to control the therapy operation of the first negative pressure wound therapy device based on an input from the user interface; and
a processing circuit configured to:
cause the wireless radio to communicate with the second negative pressure wound therapy device and receive live operational information from the second negative pressure wound therapy device, the live operational information including a current negative pressure value of the second negative pressure wound therapy device;
cause the wireless radio to communicate with the second negative pressure wound therapy device to receive information from the second negative pressure wound therapy device including an alert regarding a malfunction of the second negative pressure wound therapy device;
determine the wireless radio connection strength between the first negative pressure wound therapy device and the second negative pressure wound therapy device based on the communication between the first negative pressure wound therapy device and the second negative pressure wound therapy device;
determine a distance between the first negative pressure wound therapy device and the second negative pressure wound therapy device based on the wireless radio connection strength; and
cause the user interface to display the determined wireless radio connection strength, the distance between the first negative pressure wound therapy device and the second negative pressure wound therapy device, and the live operational information of the second negative pressure wound therapy device to the user.
|