| CPC G16H 10/40 (2018.01) [G01N 11/00 (2013.01); G01N 33/49 (2013.01)] | 20 Claims |
|
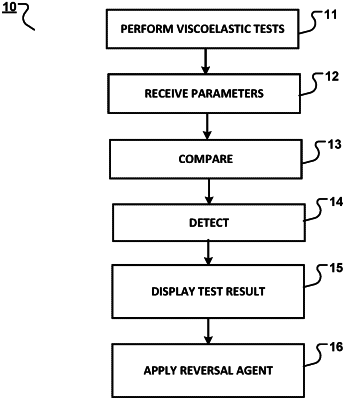
1. A method detecting a presence of one or more oral anticoagulants or intravenous (IV) direct thrombin inhibitors in a blood sample, the method comprising:
receiving parameters that are based on viscoelastic tests;
comparing the parameters to predefined threshold values, the parameters and the predefined threshold values being based on an identity of the one or more oral anticoagulants or the IV direct thrombin inhibitors; and
detecting, based on the comparing, the presence of the one or more oral anticoagulants or IV direct thrombin inhibitors in the blood sample;
wherein the viscoelastic tests are performed on portions of the blood sample to obtain the parameters, the viscoelastic tests comprising both a viscoelastic test based on Ecarin activation and a viscoelastic test with low tissue factor activation.
|