| CPC C09D 7/63 (2018.01) [B22C 9/043 (2013.01); B29C 64/124 (2017.08); B33Y 10/00 (2014.12); B33Y 70/00 (2014.12); C08F 2/50 (2013.01); C08G 59/22 (2013.01); C08G 59/24 (2013.01); C08G 59/245 (2013.01); C08G 59/687 (2013.01); C08G 65/18 (2013.01); C09D 163/00 (2013.01); B29K 2105/0005 (2013.01)] | 19 Claims |
|
1. A liquid radiation curable composition for additive fabrication comprising:
a cationically polymerizable component;
a radically polymerizable component;
an antimony-free cationic photoinitiator with an anion selected from the group consisting of PF6−, BF4−, ((CF3)2C6H3)4B−, (C6F5)4Ga−, ((CF3)2C6H3)4Ga−, trifluoromethanesulfonates, nonafluorobutanesulfonates, methanesulfonates, butanesulfonates, benzenesulfonates, p-toluenesulfonates, and PFα(Rf)β, wherein α and β are the same or different, and are integers from 1-8, and Rf contains a carbon atom and a halogen atom, and
a cation of the following general formula:
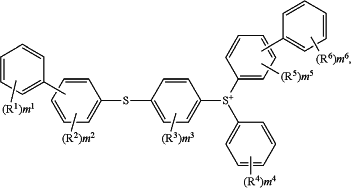 wherein R1, R2, R3, R5 and R6 each independently represent an alkyl group, a hydroxy group, an alkoxy group, an alkylcarbonyl group, an arylcarbonyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, an arylthiocarbonyl group, an acyloxy group, an arylthio group, an alkylthio group, an aryl group, a heterocyclic hydrocarbon group, an aryloxy group, an alkylsulfinyl group, an arylsulfinyl group, an alkylsulfonyl group, an arylsulfonyl group, a hydroxy(poly)alkyleneoxy group, an optionally substituted amino group, a cyano group, a nitro group, or a halogen atom,
R4 represents an alkyl group, a hydroxy group, an alkoxy group, an alkylcarbonyl group, an alkoxycarbonyl group, an acyloxy group, an alkylthio group, a heterocyclic hydrocarbon group, an alkylsulfinyl group, an alkylsulfonyl group, a hydroxy(poly)alkyleneoxy group, an optionally substituted amino group, a cyano group, a nitro group, or a halogen atom,
m1 to m6 each represent the number of occurrences of each of R1 to R6, m1, m4, and m6 each represent an integer of 0 to 5, and m2, m3, and m5 each represent an integer of 0 to 4; and
a photosensitizer possessing the following structure:
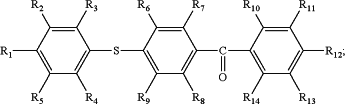 wherein R1-R14 are independently H or a C1-C8 saturated or unsaturated, branched or unbranched, substituted, or unsubstituted hydrocarbyl;
a free-radical photoinitiator; and
a UV absorber;
wherein the UV absorber comprises one or more compounds selected from the group consisting of cinnamate derivatives, salicylate derivatives, benzophenone derivatives, camphor derivatives, and dibenzoyl methane derivatives, and any combinations thereof.
|