| CPC C07D 405/14 (2013.01) [A61P 35/00 (2018.01); A61K 31/496 (2013.01); A61K 31/506 (2013.01)] | 13 Claims |
|
1. A compound having a structure according to Formula (I):
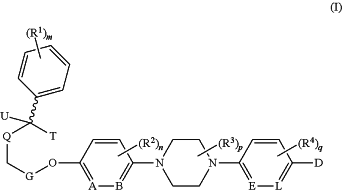 or an optically pure stereoisomer or pharmaceutically acceptable salt thereof,
wherein,
U is
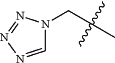 R1, R2, R3, and R4 are each independently selected from the group consisting of alkoxy, alkyl, alkynyl, amino, amido, halogen, hydroxyl, haloalkyl, perhaloalkyl, perhaloalkoxy, nitro, and cyano, wherein alkoxy, alkyl, and alkynyl are optionally substituted with halogen, hydroxyl, or nitro group;
A is CR5 or N;
B is CR6 or N;
E is CR7 or N;
L is CR8 or N;
R5, R6, R7, and R8 are each independently selected from the group consisting of hydrogen, alkoxy, alkyl, alkynyl, amino, halogen, hydroxyl, haloalkyl, perhaloalkyl, perhaloalkoxy, nitro, and cyano, wherein alkoxy, alkyl, and alkynyl are optionally substituted with halogen, hydroxyl, or nitro group;
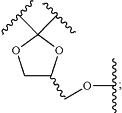
G, Q, and T together with the atom(s) to which they are attached joined together to form dioxolane
 m is an integer between 0 and 5;
n and q are each independently an integer between 0 and 2;
p is an integer between 0 and 4;
D is selected from the group consisting of:
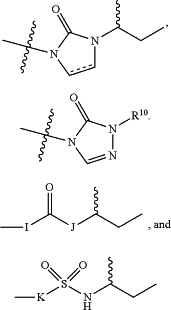 wherein,
 is a single or double bond; is a single or double bond;R10 is selected from the group consisting of hydrogen, alkyl, arylalkyl, alkoxyalkyl, arylalkoxy, alkynylalkyl, alkylalkynylalkyl, alkenylalkyl, alkylalkenylalkyl, cycloalkyl, cyanoalkyl, cycloalkylalkyl, heteroalkyl, heterocycloalkyl, heteroaryl, heteroarylalkyl, heterocycloalkylalkyl, and alkylsulfonyl, any of which can be optionally substituted with halogen, hydroxyl, or nitro group;
I is (CH2)r or NH;
J is (CH2)s or NH;
K is (CH2)t or NH; and
r, s, and t are each independently an integer between 0 and 4.
|