| CPC C07D 401/04 (2013.01) [B01J 23/44 (2013.01); B01J 23/755 (2013.01)] | 48 Claims |
|
1. A process for the synthesis of a compound of formula (I);
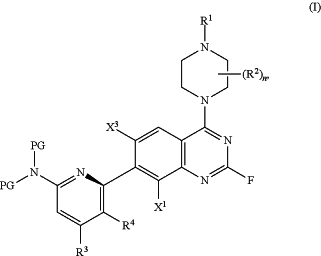 or a solvate, tautomer, stereoisomer, atropisomer, or salt thereof, wherein
X1 and X3 are each independently hydrogen or halogen;
R1 is hydrogen or PG1;
each R2 is independently halogen, cyano, unsubstituted C1-6 alkyl, unsubstituted C1-6 cyanoalkyl, or unsubstituted C1-6 haloalkyl;
R3 is hydrogen, halogen, R3A-substituted or unsubstituted C1-3 alkyl, R3A-substituted or unsubstituted C1-3 haloalkyl, or R3A-substituted or unsubstituted cyclopropyl;
R3A is halogen, OH, CN, unsubstituted C1-3 alkyl or unsubstituted C1-3 haloalkyl;
R4 is R4A_substituted or unsubstituted C1-3 haloalkyl;
R4A is unsubstituted C1-3 alkyl;
n is 1 or 2;
each PG is independently an amino protecting group; and
PG1 is an amino protecting group;
wherein the process comprises
(a) contacting a compound of formula (II)
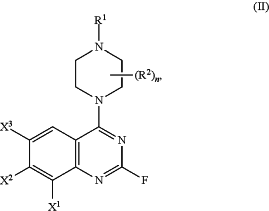 wherein X2 is halogen;
with an organomagnesium compound thereby forming a compound of formula (IIa):
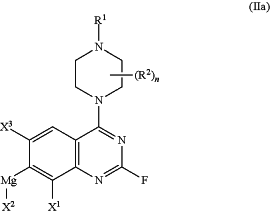 (b) transferring the compound of formula (IIa) of step (a) to a continuous stirred tank reactor (CSTR) comprising a zinc compound at a temperature of about −20° C. to 20° C. thereby synthesizing a compound of formula (IIb); and
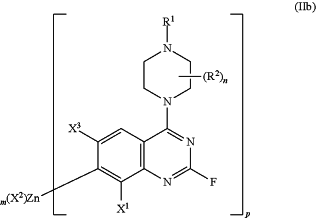 wherein m is 0, 1, or 2;
p is 1, 2, or 3; and
X2 is halogen or OPiv;
(c) contacting the compound (IIb) of step (b) with a compound of formula (III),
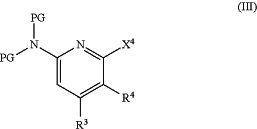 wherein X4 is halogen,
a transition metal catalyst precursor, and a chiral ligand, thereby synthesizing a compound of formula (I).
|