| CPC C07D 401/04 (2013.01) [A61K 9/0019 (2013.01); A61K 31/4178 (2013.01); A61K 31/439 (2013.01); A61K 31/44 (2013.01); A61K 31/473 (2013.01); A61K 31/496 (2013.01); A61K 31/56 (2013.01); A61K 31/573 (2013.01); A61K 31/675 (2013.01); A61K 45/06 (2013.01); A61P 1/08 (2018.01); A61P 13/10 (2018.01); A61P 25/22 (2018.01); A61P 25/24 (2018.01); C07D 213/74 (2013.01); C07D 213/76 (2013.01); C07D 213/89 (2013.01); C07F 9/025 (2013.01); C07F 9/650952 (2013.01)] | 3 Claims |
|
1. A process for synthesizing a composition comprising a chloromethyl dialkylphosphate having the following formula:
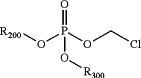 wherein:
R200 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
R300 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
each R103 is independently halogen, CN, NO2, C(O)R104, C(O)NR104R105, C(O)OR104, NR104R105, NR104C(O)R105, NR104S(O)2R105, O−, OR104, SR104, S(O)2R104, S(O)2NR104R105, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
each R104 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; and
each R105 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
wherein the process comprises the following steps:
(a) contacting a solution comprising a first C1-C6 alcohol solvent and a dialkylphosphate salt having the following formula:
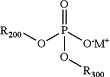 wherein:
R200 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
R300 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
each R103 is independently halogen, CN, NO2, C(O)R104, C(O)NR104R105, C(O)OR104, NR104R105, NR104C(O)R105, NR104S(O)2R105, O−, OR104, SR104, S(O)2R104, S(O)2NR104R105, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
each R104 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
each R105 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; and
M+ is K+;
with an inorganic acid selected from the group consisting of boric acid, fluoroboric acid, carbonic acid, hydrobromic acid, hydrochloric acid, hydrofluoric acid, metaphosphoric acid, nitric acid, phosphoric acid, and sulfuric acid, to form a compound having the following formula, in situ:
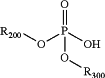 wherein:
R200 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
R300 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
each R103 is independently halogen, CN, NO2, C(O)R104, C(O)NR104R105, C(O)OR104, NR104R105, NR104C(O)R105, NR104S(O)2R15, O−1, OR104, SR104, S(O)2R104, S(O)2NR104R105, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
each R104 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; and
each R105 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
(b) contacting the compound formed in situ in step (a) above with a solution comprising a second C1-C6 alcohol solvent and a compound having the following formula:
N(C1-6 alkyl)4+OH−,
to form a compound having the following formula:
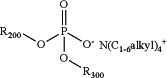 wherein:
R200 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
R300 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
each R103 is independently halogen, CN, NO2, C(O)R104, C(O)NR104R105, C(O)OR104, NR104R105, NR104C(O)R105, NR104S(O)2R105, O−1, OR104, SR104, S(O)2R104, S(O)2NR104R105, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
each R104 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; and
each R105 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
(c) contacting the compound formed in step (b) above with a solution comprising a C1-C6 aldehyde solvent or a C1-C6 ketone solvent and chloroiodomethane having the following formula:
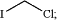 (d) removing the remaining C1-C6 aldehyde solvent or C1-C6 ketone solvent and any excess of chloroiodomethane used in step (c) above via distillation, to form a reaction mass;
(e) suspending the reaction mass formed in step (d) above in tert-butyl methyl ether (TBME);
(f) filtering the reaction mass-containing suspension formed in step (e) above, to form a filtrate;
(g) washing the filtrate formed in step (f) above with a saturated solution of sodium bicarbonate and water, to form a washed filtrate; and
(h) placing the washed filtrate formed in step (g) above under reduced pressure, to substitute any remaining solvent with acetone and form a composition comprising a chloromethyl dialkylphosphate having the following formula:
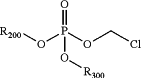 wherein:
R200 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
R300 is C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with one or more independently selected R103 substituents;
each R103 is independently halogen, CN, NO2, C(O)R104, C(O)NR104R105, C(O)OR104, NR104R105, NR104C(O)R105, NR104S(O)2R105, O−1, OR104, SR104, S(O)2R104, S(O)2NR104R105, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
each R104 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; and
each R105 is independently H, halogen, CN, NO2, alkyl, alkyl(hydroxy), alkyl(Oalkyl), alkyl(heterocycloalkyl), alkyl(aryl), alkyl(heteroaryl), alkenyl, NH2, O−1, OH, O(alkyl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl.
|