| CPC C07C 17/07 (2013.01) [B01J 19/0013 (2013.01); B01J 19/0093 (2013.01); B01J 2219/00905 (2013.01)] | 23 Claims |
|
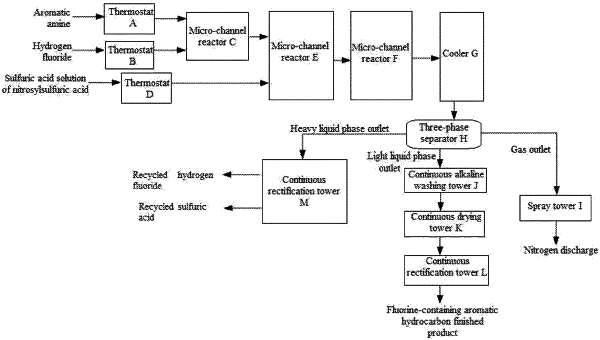
1. A full continuous flow synthesis process of fluorine-containing aromatic hydrocarbon compounds, comprising the following steps:
(1) according to a feeding ratio of aromatic amine to hydrogen fluoride, pumping aromatic amine into a thermostat A, pumping hydrogen fluoride into thermostat B, and keeping the temperatures of materials constant;
(2) allowing aromatic amine and hydrogen fluoride flowing out from the thermostats to flow into a micro-channel reactor C for a salt forming reaction to obtain a hydrofluoric acid solution of an aromatic amine hydrogen fluoride salt;
(3) pumping a sulfuric acid solution of nitrosylsulfuric acid into a thermostat D according to a feeding ratio of nitrosylsulfuric acid to aromatic amine, and keeping the temperatures of materials constant;
(4) allowing the hydrofluoric acid solution of the aromatic amine hydrogen fluoride salt flowing out from the micro-channel reactor C and the sulfuric acid solution of nitrosylsulfuric acid flowing out from the thermostat D to flow into a micro-channel reactor E for diazotization reaction to obtain an aryl diazonium salt solution;
(5) allowing the aryl diazonium salt solution flowing out from the micro-channel reactor E to flow into a micro-channel reactor F for a thermal decomposition reaction to obtain a mixture consisting of fluorine-containing aromatic hydrocarbons, hydrofluoric acid, sulfuric acid and nitrogen;
(6) allowing the mixture consisting of fluorine-containing aromatic hydrocarbons, hydrofluoric acid, sulfuric acid and nitrogen to flow through a cooler G and then enter a three-phase separator H for continuous separation, discharging nitrogen at a gas outlet of the three-phase separator H, allowing a fluorine-containing aromatic hydrocarbon crude product to flow out from a light liquid phase outlet of the three-phase separator H, and allowing a mixture of hydrofluoric acid and sulfuric acid to flow out from a heavy liquid phase outlet of the three-phase separator H;
(7) spraying nitrogen discharged from the gas outlet of the three-phase separator H in a spray tower I to remove acid, and then discharging;
(8) allowing the fluorine-containing aromatic hydrocarbon crude product flowing out from the light liquid phase outlet of the three-phase separator H to enter a continuous alkaline washing tower J for alkaline washing to remove acid, followed by dehydrating in a continuous drying tower K and rectifying in a continuous rectification tower L, so as to obtain a fluorine-containing aromatic hydrocarbon finished product; and
(9) distilling the mixture of hydrofluoric acid and sulfuric acid flowing out from the heavy liquid phase outlet of the three-phase separator H in a continuous distillation tower M to obtain recycled hydrogen fluoride and recycled sulfuric acid.
|