| CPC A61N 7/00 (2013.01) [A61K 49/223 (2013.01); A61N 5/02 (2013.01); A61N 7/02 (2013.01); C07K 14/71 (2013.01); C07K 16/2878 (2013.01); A61B 8/4218 (2013.01); A61B 8/485 (2013.01); A61B 8/546 (2013.01); A61B 2018/00613 (2013.01); A61B 2018/00994 (2013.01); A61B 2090/374 (2016.02); A61N 2007/0004 (2013.01); A61N 2007/0052 (2013.01); A61N 2007/0078 (2013.01); A61N 2007/0082 (2013.01); C07K 2317/75 (2013.01)] | 32 Claims |
|
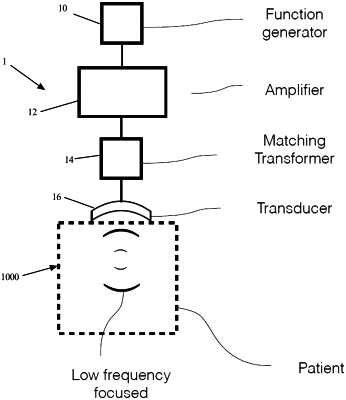
1. An acoustic priming therapy system comprising:
a processor;
one or more ultrasound transducers coupled to the processor, wherein the one or more ultrasound transducers are configured to produce one or more ultrasound beams such that the frequency waveform of the one or more ultrasound beams has a spatial peak temporal average acoustic output intensity (Ispta) of between 10 and 900 W/cm2 and a −3 dB beam profile waist of at least 5 mm; and
a probe coupled to the processor, wherein the probe is configured to monitor the patient,
wherein the processor and the ultrasound transducer are configured to scan tissue volumetrically at a rate of at least 0.5 cm3 per second,
wherein a first ultrasound transducer of the one or more ultrasound transducers is configured to produce an ultrasound beam at a frequency of 300 kHz to 3 MHz and a second ultrasound transducer of the one or more ultrasound transducers is configured to produce an ultrasound beam at a frequency of between 30 kHz and 300 kHz, and
wherein the one or more ultrasound beams is a low-Intensity Focused Ultrasound (LOFU) and induces a non-ablative stress in cells of a target treatment zone.
|