| CPC A61N 1/36125 (2013.01) [A61N 1/0553 (2013.01); A61N 1/3614 (2017.08); A61N 1/36185 (2013.01)] | 20 Claims |
|
1. A medical device, comprising:
an implantable pulse generator (IPG) configured for implantation in a patient and comprising a plurality of electrode nodes, each electrode node configured to be coupled to an electrode configured to contact a patient's brain tissue; and
control circuitry configured to:
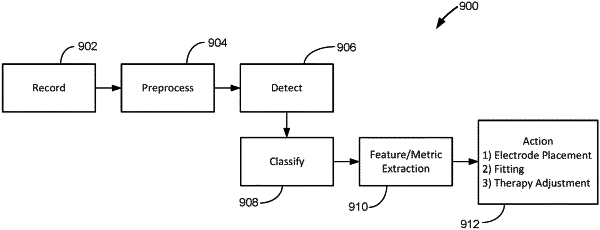
control stimulation circuitry to issue stimulation at a first one or more of the plurality of electrode nodes,
record an electrical signal at a second one or more of the plurality of electrode nodes,
classify the recorded electrical signal according to one or more classification criteria indicative of evoked resonant neural activity (ERNA) to determine if the recorded electrical signal contains ERNA,
if the recorded electrical signal does contain ERNA, extract one or more features of the ERNA,
adjust stimulation based on the one or more features, and
if the recorded electrical signal does not contain ERNA, make no adjustment based on the signal.
|