| CPC A61K 31/506 (2013.01) [A61K 31/713 (2013.01); C07K 16/2896 (2013.01)] | 8 Claims |
|
1. A method of treating a subject having skin cancer, wherein the subject is heterozygous for a Carboxypeptidase Vitellogenic Like (CPVL) variant genomic nucleic acid molecule, the method comprising administering a Carboxypeptidase Vitellogenic Like (CPVL) inhibitor to the subject;
wherein the CPVL variant genomic nucleic acid molecule comprises 7:29096102:C:T, 7:28995873:G:A, 7:29030645:C:CT, 7:29096125:A:T, 7:29066109: GT:G, 7:29064221:T:TG, 7:29120891:A:C, 7:29071905:C:CT, 7:29071905:C:G, 7:29064060:C:T, 7:29064167:AC: A, 7:29086513: T: A, 7:29064132:C:CA, 7:29066026:G:T, 7:28995813:G:A, 7:29092703:C:T, 7:29195076:C:T, 7:29120892:C:A, 7:29066070:G:A, 7:29064236:T:C, 7:29095143:C:T, 7:29095143: CT:C, 7:29071879: AG:A, 7:29064077: AT: A, 7:29064100:TG:T, 7:29030600:GGA:G, 7:29066069:TG:T, 7:29064120:G:A, 7:28995858:TA:T, 7:29096218:C:T, 7:29120903: CT:C, 7:29112720:C:T, 7:29195076:C:A, 7:29030581: TGGAA:T, 7:29096219:T:C, 7:29096142:CAA:C, 7:29071905:C:T, 7:29121003: CAG:C, 7:29096102:C:G, 7:29064120:G:GC, 7:29071772:C:A, 7:29120995:GC: G, 7:29066060:G:GT, 7:29030645:CT:C, 7:29064060:C:A, 7:29066032:CA:C, 7:29112740:G:T, 7:29120989:G:GA, 7:29112702:A:G, 7:29030604:GT:G, 7:29064150:TAA:T, 7:29064087: A:AG, 7:29064093: GC:G, 7:29112740:G:C, 7:29064060:CCTTAT:C, 7:28995830; GCT:G, 7:29064221: TG:T, 7:29086483:C:T, 7:29030760:C:CTGAAA, 7:29096207:T:TCTGG, 7:29066114:TC:T, 7:29072425:T:A, 7:29112817:C:A, 7:29064115:TTC:T, 7:29086488:C:T, 7:29095087:A:T, 7:28995863:C:T, 7:29092645:C:A, 7:29096130:G:A, 7:29112766:C:T, 7:29112757:T:C, 7:29096177:C:T, 7:28995863:C:A, 7:29066115:C:A, 7:29030726:C:T, 7:29030584: A:T, 7:29072407:T:C, 7:29064135:C:G, 7:29030749:T:C, 7:29095089: T:G, 7:29030710:G:C, 7:28995876:T: A, 7:29066063: A:T, 7:29064068: T:A, 7:29112774:C:T, 7:29071894:C:T, 7:29096198:G:A, 7:29112810:C:T, 7:29120923:G:C, 7:29086539:T:C, 7:29072410:T:C 7:29120922:G:C, 7:28995851:G:C, 7:29030642:A:T, 7:29096199:G:C, 7:29096169:C:T, 7:29030738:G:C, 7:29064134: A:G, 7:29064173: A:G, 7:28995809: A:C, 7:29096199:G:A, 7:29096150:C:A, 7:29030599:C:T, 7:29072321:C:T, 7:29030737: A:C, 7:29030578:T:C, 7:29096172:G:A, 7:29092663: A:G, 7:29030682:C:A, 7:29095097:T:C, 7:29086497:T:G, 7:29092632:T:A, 7:29030611:G:A, 7:28995872:C:T, 7:28995816:T:G, 7:29092666:C:T, 7:29066097:C:T, 7:28995859: A:T, 7:29086486:C:G, 7:29086549:C:T, 7:29072416:G:A, 7:29096136:G:C, 7:29095103: A:G, 7:29092638:G:A, 7:29092660:C:T, 7:29066058:C.G, 7:29072329:A:G, 7:29030727:G:C, 7:29095088:T:A, 7:29064125:T:C, 7:29086515:T.C, 7:29064122: A:G, 7:29064090: A:G, 7:29096171:G:A, 7:29086548:G:T, 7:28995873:G:C, 7:29066117:A:G, 7:29064101:G:A, 7:29030740:T:C, 7:29095089: T:C, 7:29064164:C:T, 7:29071803:G:C, 7:29064168:C:T, 7:28995864:C:T, 7:29096144: A:G, 7:29096172:G:T, 7:29071780:G:C, 7:29112725:G:T, 7:29092680:G:A, 7:29066071:G:C, 7:29096184:G:C 7:29096112:T: A, 7:29072423: A:G, 7:29030722:G:A, 7:29064098: A:C, 7:28995852:G:A, 7:28995846:C:T, 7:29030609:C:A, 7:29112754:C:T, 7:29092683: A:C, 7:29112732:T:C, 7:29096139:C:T, 7:29096136:G:A, 7:29112719:C:A, 7:29072347:T:G, 7:29086548:G:A, 7:29064092:G:T, 7:29112750:T:C, 7:29112735:C:G, 7:29096121:C:A, 7:28995814: A:T, 7:29066108: A:G, 7:29092693:C:T, 7:29096169:C:G, 7:29095101:G:A, 7:29064212:T:C, 7:29120929:G: A, 7:29030705:G: A, 7:29095086:G:T, 7:28995812:C:T, 7:29096143: A:C, 7:29030577:C:G, 7:28995822:T:C, 7:29096174:C:G, 7:29072380: T:C, 7:29066105: A:G, 7:29064213: A:T, 7:29096138:T:C, 7:29064174:T:C, 7:29086498: A:G, 7:29064152: A:G, 7:29096159:G: A, 7:29096160: A:G, 7:29030750: A:G, 7:29071781:C:T, 7:29072339:C:T, 7:29030600:G:A, and/or 7:29096120:A:G; and
wherein the CPVL inhibitor comprises:
i) an inhibitory nucleic acid molecule that hybridizes to a CPVL nucleic acid molecule;
ii) hydroxymethyl(N-methyliminodiacetic acid)boronate (hydroxymethyl(MIDA)boronate), azidomethyl(N-methyliminodiacetic acid)boronate (azidomethyl(MIDA)boronate), or an α-functionalized alkyl(MIDA)boronate compound;
iii) a compound selected from the group consisting of
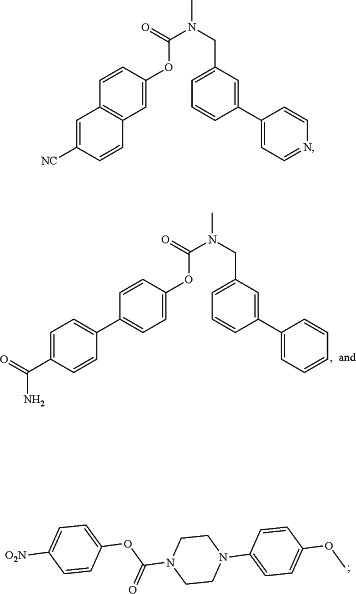 or
iv) an immune checkpoint inhibitor.
|