| CPC A61K 31/415 (2013.01) | 20 Claims |
|
1. A method of treating transthyretin amyloidosis (ATTR) cardiomyopathy in a subject in need thereof, the method comprising orally administering to a subject in need thereof a total daily dosage of about 1,600 milligrams (mg) of Compound 1 in HCl salt form or an equivalent amount of Compound 1 in freebase or in a different salt form, wherein Compound 1 has the Formula:
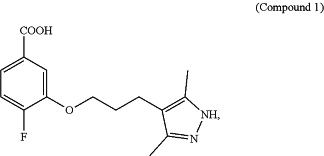 wherein Compound 1 in HCl salt form or an equivalent amount of Compound 1 in freebase or in a different salt form is administered twice daily.
|