| CPC A61K 31/41 (2013.01) [A61K 9/0019 (2013.01); A61K 47/40 (2013.01)] | 5 Claims |
|
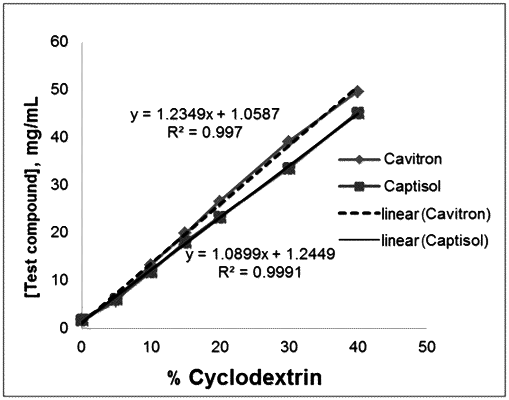
1. A parenteral liquid formulation comprising as a sole active ingredient a carbamate compound which is carbamic acid (R)-1-(2-chlorophenyl)-2-tetrazol-2-yl-ethyl ester of the following Formula 2, or a pharmaceutically acceptable salt, solvate or hydrate of the compound of Formula 2; and a cyclodextrin derivative:
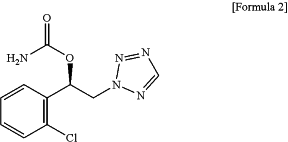 wherein the cyclodextrin derivative is 2-hydroxypropyl-β-cyclodextrin or sulfobutyl ether-β-cyclodextrin, and
wherein a weight ratio of the active ingredient to the cyclodextrin derivative is 1:5 to 1:40; and
wherein the carbamate compound of Formula 2 and the cyclodextrin form an inclusion complex.
|