| CPC H01M 4/525 (2013.01) [C01G 53/00 (2013.01); C01G 53/006 (2013.01); C01G 53/04 (2013.01); C01G 53/50 (2013.01); H01M 4/505 (2013.01); H01M 10/0525 (2013.01); C01P 2002/74 (2013.01); C01P 2004/03 (2013.01); C01P 2004/50 (2013.01); C01P 2004/51 (2013.01); C01P 2004/54 (2013.01); C01P 2004/61 (2013.01); C01P 2004/64 (2013.01); C01P 2006/11 (2013.01); C01P 2006/14 (2013.01); C01P 2006/16 (2013.01); C01P 2006/40 (2013.01); H01M 2004/028 (2013.01)] | 9 Claims |
|
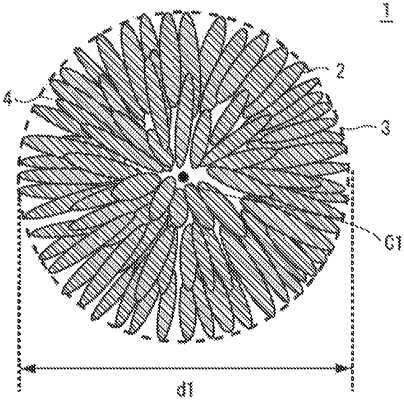
1. A nickel-manganese composite hydroxide represented by General Formula (1): NixMnyMz(OH)2+α (in Formula (1), M is at least one additional element selected from Co, Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, Fe, and W; and x, y, z, and α satisfy 0.1≤x≤0.8, 0.1≤y≤0.6, 0≤z≤0.8, x+y+z=1.0, and 0≤α≤0.4) and containing a secondary particle formed of a plurality of flocculated primary particles, wherein
the primary particles have an aspect ratio of at least 3, and at least some of the primary particles are disposed radially in a direction from a central part of the secondary particle to an outer circumference thereof, and
the secondary particle has a ratio (I(101)/I(001)) of a diffraction peak intensity I(101) of a 101 plane to a diffraction peak intensity I(001) of a 001 plane, measured by an X-ray diffraction measurement, of up to 0.15.
|
|
5. A method for producing a nickel-manganese composite hydroxide represented by General Formula (1): NixMnyMz(OH)2+α (in Formula (1), M is at least one additional element selected from Co, Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, Fe, and W; and x, y, z, and α satisfy 0.1≤x≤0.8, 0.1≤y≤0.6, 0≤z≤0.8, x+y+z=1.0, and 0≤α≤0.4) and containing a secondary particle formed of a plurality of flocculated primary particles,
the method comprising a crystallization process of forming a nickel-manganese composite hydroxide by neutralizing a salt containing at least nickel and a salt containing at least manganese in an aqueous reaction solution, wherein
in the crystallization process, a dissolved nickel concentration in the aqueous reaction solution is controlled in a range of at least 300 mg/L and up to 1,500 mg/L, a dissolved oxygen concentration is controlled in a range of at least 0.5 mg/L and up to 3.5 mg/L, and a stirring power applied to the aqueous reaction solution is controlled in a range of at least 4 kW/m3 and up to 8 kW/m3.
|