| CPC G16H 40/40 (2018.01) | 22 Claims |
|
1. A method comprising:
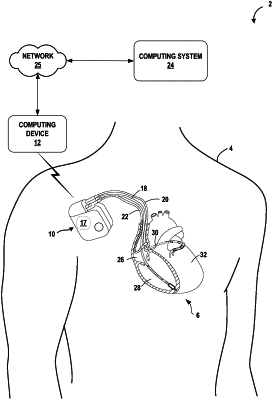
receiving, by processing circuitry of a computing device, diagnostic data of a medical device implanted in a patient;
determining, by the processing circuitry, a use case associated with analyzing the diagnostic data out of a plurality of use cases for analyzing the diagnostic data, wherein the plurality of use cases include post-surgical follow-up monitoring of the medical device, routine follow-up monitoring of the medical device, and exception-based follow-up monitoring of the medical device;
storing, by the processing circuitry in memory of the computing device, a value indicative of whether to route the diagnostic data to a computing system for analysis;
in response to determining, based on the value stored in the memory, to refrain from routing the diagnostic data to the computing system, determining, by the processing circuitry and based at least in part on the use case, one or more device characteristics data to be compared against the diagnostic data; and
analyzing, by the processing circuitry and based at least in part on comparing the diagnostic data with the one or more device characteristics data, the diagnostic data to determine an operating status of the medical device.
|