| CPC G01N 33/56983 (2013.01) [G01N 21/6428 (2013.01); G01N 2021/6439 (2013.01); G01N 2333/165 (2013.01)] | 21 Claims |
|
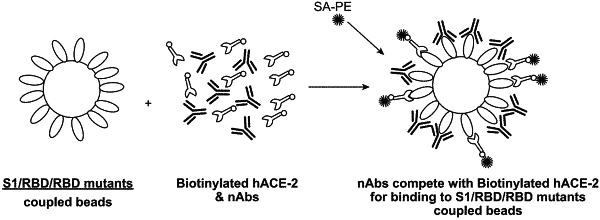
1. A substrate comprising a population of particles or beads comprising two or more separate subpopulations of particles or beads, each subpopulation of particles or beads being distinguishable by a specific detectable parameter and each subpopulation of beads comprising a distinct SARS-CoV-2 spike protein variant or fragment thereof immobilized thereon and, optionally, a further subpopulation of beads comprising a SARS-CoV-2 spike protein or fragment thereof immobilized thereon.
|