| CPC C07D 487/04 (2013.01) [C07D 471/04 (2013.01); C07D 513/04 (2013.01); C07D 519/00 (2013.01)] | 18 Claims |
|
1. A compound of Formula IV:
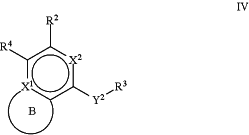 or a pharmaceutically acceptable salt, stereoisomer, or tautomer thereof,
wherein:
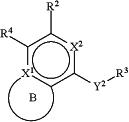 is:
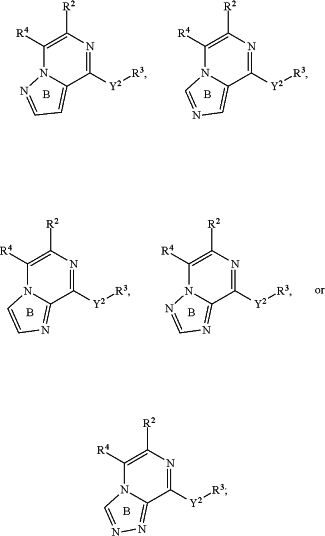 wherein ring B is optionally substituted on any available carbon with one or two substituents independently selected from the group consisting of C1-C6 alkyl, CH2F, CHF2, CF3, (CH2)nNH2, (CH2)nOH, heterocyclyl, and heteroaryl;
R2 is H, halogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, NR5R6, OH, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, or heterocyclyl;
wherein the heterocyclyl contains 1, 2, 3, 4, or 5 heteroatoms independently selected from the group consisting of nitrogen, phosphorous, oxygen, and sulfur;
wherein the heterocyclyl is not attached via a nitrogen atom; and
wherein the C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, or heterocyclyl is optionally substituted with one or more substituents independently selected from the group consisting of halogen, CN, NO2, NR5R6, NR5S(O)R6, NR5S(O)NR5R6, NR5S(O)2R6, NR5S(O)2NR5R6, OR5, ═O, SR5, S(O)R5, S(O)NR5R6, S(O)2R5, S(O)2NR5R6, heterocyclyl, aryl, heteroaryl, and R5;
Y2 is —NRa', —NRaC(O)—, —NRaC(O)NRa—, —NRaC(O)O—, —NRaC(S)—, —NRaC(S)NRa—, or —NRaS(O)2—, wherein the bond on the left side of Y2 is bound to the ring and the bond on the right side of Y2 is bound to R3;
R3 and Ra, together with the atom(s) to which they are attached, form a monocyclic or polycyclic 3- to 12-membered heterocyclyl or a spirocyclic 5- to 12-membered heterocyclyl;
wherein the monocyclic or polycyclic 3- to 12-membered heterocyclyl or spirocyclic 5- to 12-membered heterocyclyl is optionally substituted with one or more substituents independently selected from the group consisting of C1-C6 alkyl, CH2F, CHF2, CF3, (CH2)nNH2, (CH2)nOH, ═O, heterocyclyl, and heteroaryl;
R4 is:
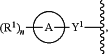 wherein:
Y1 is a direct bond, —CH2—, —C(═CH2)—, —NH—, —S—, —S(O)—, —S(O)2—, or —S(O)2NH—;
ring A is a monocyclic or polycyclic 5- to 12-membered cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; and
each R1 is independently H, D, halogen, CN, NO2, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C(O)R5, C(O)OR5, NR5R6, NR5S(O)R6, NR5S(O)NR5R6, NR5S(O)2R6, NR5S(O)2NR5R6, OR5, SR5, S(O)R5, S(O)NR5R6, S(O)2R5, S(O)2NR5R6, C3-C8 cycloalkyl, or C4-C8 cycloalkenyl;
wherein each C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C8 cycloalkyl, and C4-C8 cycloalkenyl is optionally and independently substituted with one or more substituents independently selected from the group consisting of halogen, CN, NO2, NR5R6, NR5S(O)R6, NR5S(O)NR5R6, NR5S(O)2R6, NR5S(O)2NR5R6, OR5, ═O, SR5, S(O)R5, S(O)NR5R6, S(O)2R5, S(O)2NR5R6, heterocyclyl, aryl, heteroaryl, and R5;
each R5 is independently H, D, halogen, CN, NO2, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, NR7R8, OR7, SR7, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, or a monocyclic or polycyclic 3- to 12-membered heterocyclyl;
each R6 is independently H, D, halogen, CN, NO2, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, NR7R8, OR7, SR7, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, or a monocyclic or polycyclic 3- to 12-membered heterocyclyl;
each R7 is independently H, D, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, or a monocyclic or polycyclic 3- to 12-membered heterocyclyl;
wherein each C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, and 3- to 12-membered monocyclic or polycyclic heterocyclyl is optionally and independently substituted with one or more substituents independently selected from the group consisting of CN, NO2, NH2, OH, and SH;
each R8 is independently H, D, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, or a monocyclic or polycyclic 3- to 12-membered heterocyclyl;
wherein each C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, and 3- to 12-membered monocyclic or polycyclic heterocyclyl is optionally and independently substituted with one or more substituents independently selected from the group consisting of CN, NO2, NH2, OH, and SH; and
each n is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
with the provisos that:
(1) the heteroaryl of ring A is not furanyl or thiophenyl; and
(2) Ra and R3, together with the atom(s) to which they are attached, do not form an optionally substituted piperazinyl.
|
|
16. A pharmaceutical composition comprising a pharmaceutically acceptable carrier and a compound of claim 1, or a pharmaceutically acceptable salt, stereoisomer, or tautomer thereof.
|