| CPC A61K 31/4439 (2013.01) [A61K 31/407 (2013.01); A61K 31/421 (2013.01); A61K 31/422 (2013.01); A61K 31/427 (2013.01); A61K 31/431 (2013.01); A61K 31/438 (2013.01); A61K 31/4375 (2013.01); A61K 31/4709 (2013.01); A61K 31/5377 (2013.01); A61K 31/5383 (2013.01); A61P 31/04 (2018.01); C07D 263/26 (2013.01); C07D 413/04 (2013.01); C07D 413/06 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 417/12 (2013.01); C07D 471/04 (2013.01); C07D 491/048 (2013.01); C07D 495/04 (2013.01); C07D 498/04 (2013.01)] | 14 Claims |
|
1. A method of treating a methicillin-resistant S. aureus infection or a methicillin-resistant S. epidermidis infection in a patient by administering to a patient in need thereof a synergistic combination of a beta-lactam antibiotic, wherein the beta-lactam antibiotic is cephalexin, imipenem, or dicloxacillin; a pharmaceutically acceptable carrier; and a compound of structural formula I:
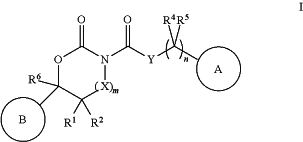 or a pharmaceutically acceptable salt thereof; wherein
A is selected from the group consisting of:
(1) aryl,
(2) heteroaryl,
(3) —O-aryl, and
(4) —O-heteroaryl,
wherein aryl and heteroaryl are unsubstituted or substituted with 1-5 substituents selected from Ra;
B is selected from the group consisting of:
(1) aryl, and
(2) heteroaryl,
wherein aryl and heteroaryl are substituted, and wherein aryl and heteroaryl are substituted with 0-4 substituents selected from Rb1 and 0-1 substituents selected from Rb2;
X is —CR8R9;
Y is selected from the group consisting of:
(1) NR3, and
(2) —CR10R11;
R1 is selected from the group consisting of:
(1) —C1-6alkyl, and
(2) —(CH2)p—OH,
wherein CH2 and alkyl are unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
R2 is selected from the group consisting of:
(1) hydrogen, and
(2) —C1-6alkyl,
wherein alkyl is unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl, or R1 and R2 together with the carbon atom they are attached to form a C3-6cycloalkyl ring, wherein the cycloalkyl ring is unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
R3 is selected from the group consisting of:
(1) hydrogen, and
(2) —C1-6alkyl,
wherein alkyl is unsubstituted or substituted with one to five substituents selected from —C1-6alkyl;
each R4 is independently selected from the group consisting of:
(1) hydrogen,
(2) halogen,
(3) —C1-6alkyl, and
(4) —(CH2)p—OH,
wherein CH2 and alkyl are unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
each R5 is independently selected from the group consisting of:
(1) hydrogen,
(2) halogen,
(3) —C1-6alkyl, and
(4) —(CH2)p—OH,
wherein CH2 and alkyl are unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
R6 is selected from the group consisting of:
(1) hydrogen,
(2) —C1-6alkyl, and
(3) —(CH2)p—OH,
wherein CH2 and alkyl are unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
R8 is selected from the group consisting of:
(1) hydrogen, and
(2) —C1-6alkyl,
wherein alkyl is unsubstituted or substituted with 1-2 substituents selected from: halogen, C1-6alkyl, and —OC1-6alkyl;
R9 is selected from the group consisting of:
(1) hydrogen, and
(2) —C1-6alkyl,
wherein alkyl is unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
R10 is selected from the group consisting of:
(1) hydrogen,
(2) —C1-6alkyl, and
(3) —(CH2)p—OH,
wherein CH2 and alkyl are unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
R11 is selected from the group consisting of:
(1) hydrogen,
(2) —C1-6alkyl, and
(3) —(CH2)p—OH,
wherein CH2 and alkyl are unsubstituted or substituted with 1-2 substituents selected from: halogen, —C1-6alkyl, and —OC1-6alkyl;
each Ra is independently selected from the group consisting of:
(1) halogen,
(2) —C1-6alkyl,
(3) —OC1-6alkyl,
(4) —OC2-6alkenyl,
(5) —OH,
(6) oxo,
(7) —CN,
(8) —NO2,
(9) —NRcRd,
(10) —CH2NRcRd,
(11) —SO2C1-6alkyl,
(12) —C3-6cycloalkyl,
(13) —C2-6cycloheteroalkyl,
(14) aryl, and
(15) heteroaryl,
wherein —CH2, alkyl, alkenyl, cycloalkyl, cycloheteroalkyl, aryl and heteroaryl are unsubstituted or substituted with 1-4 substituents selected from: halogen, —C1-6alkyl, —OC1-6alkyl, and —CO2C1-6alkyl;
each Rb1 is independently selected from the group consisting of:
(1) halogen,
(2) —C1-6alkyl,
(3) —C2-6alkenyl,
(4) —CN,
(5) —NO2,
(6) —NRcRd,
(7) —SO2C1-6alkyl,
(8) —C3-6cycloalkyl,
(9) —C2-6cycloheteroalkyl,
(10) aryl, and
(11) heteroaryl,
wherein alkyl, alkenyl, cycloalkyl, cycloheteroalkyl, aryl and heteroaryl are unsubstituted or substituted with 1-4 substituents selected from: halogen, —C1-6alkyl, —OC1-6alkyl, and —CO2C1-6alkyl;
each Rb2 is independently selected from the group consisting of:
(1) —OC1-6alkyl,
(2) —OC2-6alkenyl,
(3) —OH,
(4) oxo,
wherein alkyl, alkenyl are unsubstituted or substituted with 1-4 substituents selected from: halogen, —C1-6alkyl, —OC1-6alkyl, and —CO2C1-6alkyl;
each Rc is independently selected from the group consisting of:
(1) hydrogen,
(2) C1-6alkyl,
(3) C2-6alkenyl,
(4) C1-6alkyl-OH,
(5) C3-6cycloalkyl,
(6) C(O)C1-6alkyl, and
(7) SO2C1-6alkyl,
wherein alkyl, alkenyl and cycloalkyl are unsubstituted or substituted with one to three substituents selected from halogen, —C1-6alkyl, —OC1-6alkyl, and —CO2C1-6alkyl;
each Rd is independently selected from the group consisting of:
(1) hydrogen,
(2) C1-6alkyl,
(3) C2-6alkenyl,
(4) C1-6alkyl-OH,
(5) C3-6cycloalkyl,
(6) C(O)C1-6alkyl, and
(7) SO2C1-6alkyl,
wherein alkyl, alkenyl and cycloalkyl are unsubstituted or substituted with one to three substituents selected from halogen, —C1-6alkyl, —OC1-6alkyl, and —CO2C1-6alkyl,
or Rc and Rd together with the nitrogen atom they are attached to form a C4-8cycloheteroalkyl ring, wherein the C4-8cycloheteroalkyl ring is unsubstituted or substituted with 1-4 substituents selected from halogen, —C1-6alkyl, —OC1-6alkyl, and —CO2C1-6alkyl;
m is 0 or 1;
n is 0, 1, 2 or 3; and
p is 0, 1, 2, 3, 4, 5 or 6.
|