| CPC A61K 9/0021 (2013.01) [A61K 31/445 (2013.01); A61K 47/36 (2013.01); A61M 37/0015 (2013.01); A61M 2037/0023 (2013.01)] | 13 Claims |
|
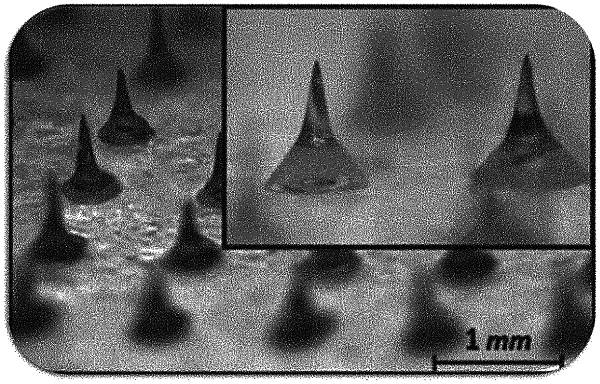
1. A soluble microneedle comprising a homogeneous composition of donepezil or a pharmaceutically acceptable salt thereof and hyaluronic acid or a pharmaceutically acceptable salt thereof,
wherein a weight ratio of the donepezil or the pharmaceutically acceptable salt thereof and the hyaluronic acid or a pharmaceutically acceptable salt thereof,
is 1:1.5 to 1:2.05;
wherein the content of the donepezil or the pharmaceutically acceptable salt thereof is 43 wt % or less of the total weight of the composition.
|