| CPC H10K 85/6572 (2023.02) [C07D 209/86 (2013.01); C07D 265/38 (2013.01); C07D 401/04 (2013.01); C07D 401/10 (2013.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 413/04 (2013.01); C07D 487/04 (2013.01); C09K 11/06 (2013.01); H10K 85/615 (2023.02); H10K 85/622 (2023.02); H10K 85/623 (2023.02); H10K 85/624 (2023.02); H10K 85/626 (2023.02); H10K 85/631 (2023.02); H10K 85/633 (2023.02); H10K 85/636 (2023.02); H10K 85/654 (2023.02); H10K 85/657 (2023.02); H10K 85/6576 (2023.02); C09K 2211/1018 (2013.01); H10K 50/11 (2023.02); H10K 2101/10 (2023.02)] | 20 Claims |
|
1. An organic light emitting device (OLED) comprising:
an anode;
a cathode; and
an organic layer, disposed between the anode and the cathode, the organic layer comprising a first compound selected from the group consisting of Formulae BB, BC, BD, BE, BF, and BG
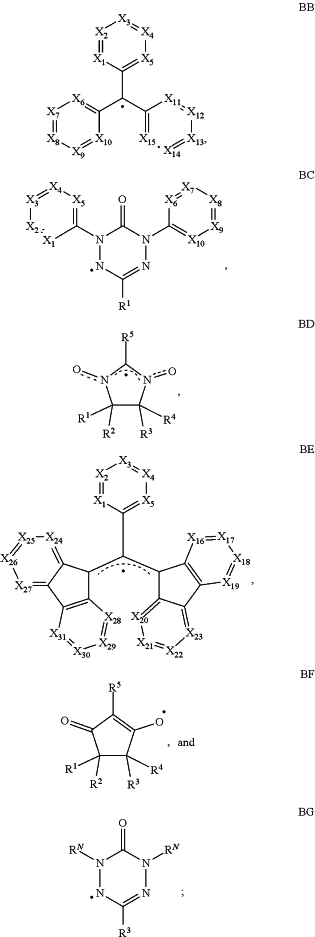 wherein
X1 to X5 are independently selected from CRA or N;
X6 to X10 are independently selected from CRB or N;
X11 to X15 are independently selected from CRC or N;
X16 to X23 are independently selected from CRD or N;
X24 to X31 are independently selected from CRE or N;
RA, RB, RC, RD, and RE independently represent mono to the maximum allowable substitution, or no substitution;
each R1 to R5, RA, RB, RC, RD, and RE are independently selected from the group consisting of hydrogen, deuterium, halide, alkyl, cycloalkyl, heteroalkyl, arylalkyl, alkoxy, aryloxy, amino, silyl, alkenyl, cycloalkenyl, heteroalkenyl, alkynyl, aryl, heteroaryl, acyl, carbonyl, carboxylic acids, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl, phosphino, and combinations thereof; or optionally, any two adjacent R1 to R4, or any two adjacent RA, RB, RC, RD, and RE, can join to form a ring;
each RN is independently selected from the group consisting of hydrogen, deuterium, halogen, alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, arylalkyl, alkoxy, aryloxy, amino, silyl, alkenyl, cycloalkenyl, heteroalkenyl, alkynyl, aryl, heteroaryl, acyl, carboxylic acid, ether, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl, phosphino, and combinations thereof;
wherein at least one of R1 to R5, RA, RB, RC, RD, or RE includes a polycyclic group selected from the group consisting of:
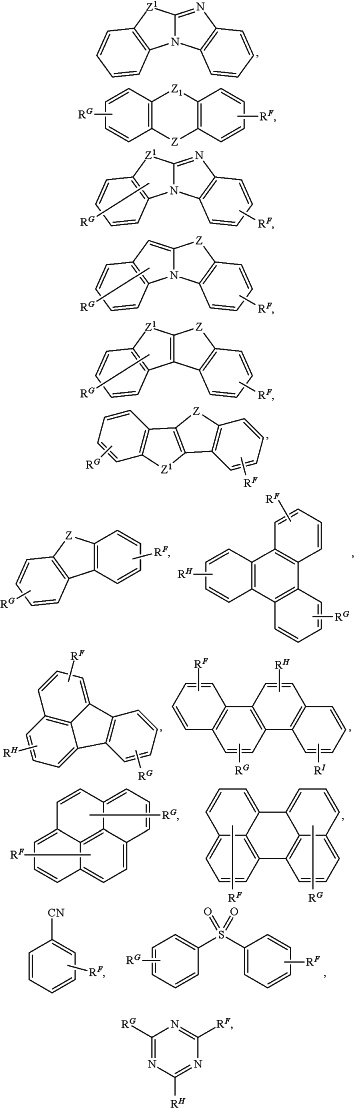 and any aza-analogue of each thereof, wherein the polycyclic group is optionally substituted with RP, wherein RP is selected from the group consisting of deuterium, halogen, alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, arylalkyl, alkoxy, aryloxy, amino, silyl, alkenyl, cycloalkenyl, heteroalkenyl, alkynyl, aryl, heteroaryl, acyl, carboxylic acid, ether, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl, phosphino, and combinations thereof,
wherein RF to RI are independently selected from the group consisting of hydrogen, deuterium, halogen, alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, arylalkyl, alkoxy, aryloxy, amino, silyl, alkenyl, cycloalkenyl, heteroalkenyl, alkynyl, aryl, heteroaryl, acyl, carboxylic acid, ether, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl, phosphino, and combinations thereof; and
Z and Z1 are independently selected from the group consisting of O, S, Se, NRN, CR′CR″, SiR′R″, and GeR′R″, wherein R′ and R″ are independently RN;
with the proviso that the following compounds are excluded
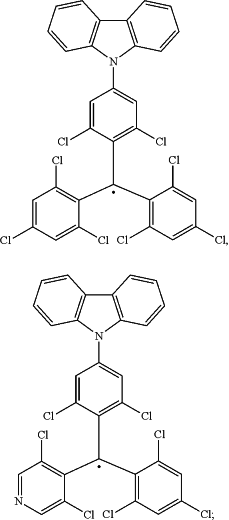 wherein the organic layer further comprises a second compound, wherein the second compound is capable of emitting light by fluorescence or thermally activated delayed fluorescence; and
wherein the organic layer further comprises a host.
|