| CPC C08G 59/245 (2013.01) [C08G 59/063 (2013.01); C08G 59/306 (2013.01); C08G 59/3218 (2013.01); C08G 59/3227 (2013.01); C08G 59/3281 (2013.01); C08G 59/5026 (2013.01); C08G 59/687 (2013.01); C08J 11/26 (2013.01); C08J 11/28 (2013.01); C08J 2363/00 (2013.01); C08L 63/00 (2013.01)] | 10 Claims |
|
1. A process for preparing an epoxy resin component having a structural Formula I and an epoxy resin component having a structural Formula II for an epoxy resin system, the process comprising:
reacting an amino alcohol with an acid compound to form an amino alcohol salt,
reacting the amino alcohol salt with a compound having a structural Formula III, a compound having a structural Formula IV or a compound having a structural Formula V in the presence of an acidic catalyst to obtain an intermediate compound,
reacting the obtained intermediate compound with a first base to obtain an amino compound; and
epoxidizing the amino compound with an epihalohydrin in the presence of a second base to obtain the epoxy resin component having the structural Formula I and the epoxy resin component having the structural Formula II, wherein,
the compound having the structural Formula III is represented by:
 wherein
R1′ and R2′ are independently hydrogen, alkyl, alkenyl, alkynyl, methylene, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heterocyclic, heterocycloalkyl, cycloalkenyl, heteroaryl, alkoxy, alkoxyaryl, alkoxy alkyl, or aryloxy,
the compound having the structural Formula IV is represented by:
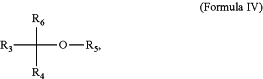 R5 is independently hydrogen, alkyl, aryl, aralkyl, alkenyl, or alkynyl;
R6, R3, and R4 are independently hydrogen, alkyl, alkenyl, alkynyl, methylene, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heterocyclic, heterocycloalkyl, cycloalkenyl, heteroaryl, alkoxy, alkoxyaryl, alkoxy alkyl, or aryloxy,
the compound having the structural Formula V is represented by:
 wherein
R7, R8, R9, and R10 are independently hydrogen, alkyl,
alkenyl, alkynyl, methylene, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heterocyclic, heterocycloalkyl, cycloalkenyl, heteroaryl, alkoxy, alkoxyaryl, alkoxy alkyl, or aryloxy,
wherein the epoxy resin component having the structural Formula I and the structural Formula II is:
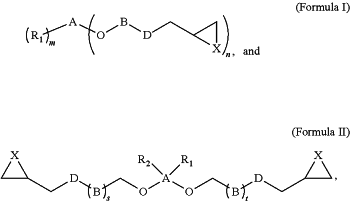 respectively,
if m=0, then n=4,
if m=1 then n=3, or
if m=2 then n=2
A is carbon or silicon,
D is oxygen or nitrogen or carboxylic group,
X is oxygen or sulfur,
s and t is independently from 1 to 20,
R1 and R2 is independently hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heterocyclic, heterocycloalkyl, cycloalkenyl, heteroaryl, alkoxyaryl, or alkoxy alkyl,
B is independently arylene, arylene ethers, alkylene-arylene, alkylene-arylene alkylene, alkenylene-arylene, alkenylene-arylene alkenylene, alkylene-arylene-alkenylene, alkynylene arylene, alkynylene-arylene-alkynylene, heteroarylene, alkylene-heteroarylene, alkylene-heteroarylene-alkylene, alkenylene-heteroarylene, alkenylene-heteroarylene-alkenylene, alkylene-heteroarylene-alkenylene, alkynylene heteroarylene, alkynylene-heteroarylene-alkynylene, alkylene, alkylene-hetero-alkylene, alkenylene, alkenylene-hetero-alkenylene, alkylene-hetero-alkenylene, alkynylene, cycloalkylene, alkylene-cycloalkylene, alkylene-cycloalkylene alkylene, alkenylene-cycloalkylene, alkenylene cycloalkylene-alkenylene, alkylene-cycloalkylene alkenylene, alkynylene-cycloalkylene, alkynylene cycloalkylene-alkynylene, heterocycloalkylene, alkylene heterocycloalkylene, alkylene-heterocycloalkylene alkylene, alkenylene-heterocycloalkylene, alkenylene heterocycloalkylene-alkenylene, alkylene heterocycloalkylene-alkenylene, alkynylene heterocycloalkylene, alkynylene-heterocycloalkylene alkynylene, cycloalkenylene, alkylene-cycloalkenylene, alkylene-cycloalkenylene-alkylene, alkenylene-cycloalkenylene, alkenylene-cycloalkenylene-alkenylene, alkylene cycloalkenylene-alkenylene, alkynylene-cycloalkenylene, alkynylene-cycloalkenylene-alkynylene, heterocycloalkenylene, alkylene-heterocycloalkenylene, alkylene-hetero cycloalkenylene-alkylene, alkenylene-heterocycloalkenylene, alkenylene-heterocycloalkenylene-alkenylene, alkylene-heterocycloalkenylene-alkenylene, alkynylene heterocycloalkenylene, alkynylene-heterocycloalkenylene, or alkynylene.
|