| CPC C07K 16/36 (2013.01) [A61P 7/04 (2018.01); C07K 16/44 (2013.01); G01N 33/86 (2013.01); G01N 33/94 (2013.01); A61K 2039/505 (2013.01); C07K 2317/21 (2013.01); C07K 2317/55 (2013.01); C07K 2317/565 (2013.01); C07K 2317/92 (2013.01)] | 18 Claims |
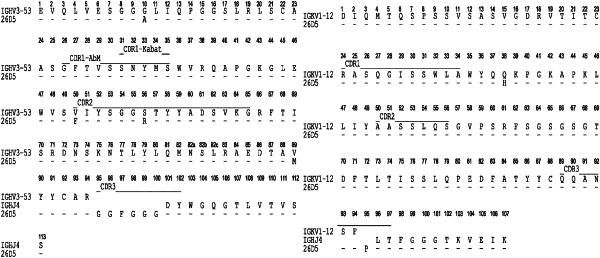
|
1. An isolated antigen binding peptide comprising a heavy chain variable region (VH) and a light chain variable region (VL), wherein the VH comprises three complementarity determining regions (CDRs): VH-CDR1, VH-CDR2, and VH-CDR3 and the VL comprises three CDRs: VL-CDR1, VL-CDR2, and VL-CDR3, wherein the amino acid sequences of the VH-CDR1, VH-CDR2, VH-CDR3, VL-CDR1, VL-CDR2, and VL-CDR3, respectively comprise the sequences selected from the group consisting of:
(a) SEQ ID NOs: 1, 13, 23, 29, 38, and 44, respectively;
(b) SEQ ID NOs: 1, 14, 23, 29, 38, and 45, respectively;
(c) SEQ ID NOs: 1, 13, 24, 30, 38, and 45, respectively;
(d) SEQ ID NOs: 1, 13, 24, 29, 39, and 45, respectively;
(e) SEQ ID NOs: 1, 14, 25, 29, 38, and 46, respectively;
(f) SEQ ID NOs: 2, 13, 26, 31, 40, and 47, respectively;
(g) SEQ ID NOs: 3, 15, 24, 32, 40, and 47, respectively;
(h) SEQ ID NOs: 4, 16, 24, 29, 38, and 46, respectively;
(i) SEQ ID NOs: 5, 15, 24, 29, 38, and 46, respectively;
(j) SEQ ID NOs: 1, 14, 24, 29, 38, and 46, respectively;
(k) SEQ ID NOs: 6, 13, 24, 31, 40, and 47, respectively;
(l) SEQ ID NOs: 3, 15, 24, 32, 41, and 48, respectively;
(m) SEQ ID NOs: 1, 14, 24, 33, 38, and 49, respectively;
(n) SEQ ID NOs: 1, 14, 26, 29, 38, and 46, respectively;
(o) SEQ ID NOs: 7, 17, 26, 29, 38, and 46, respectively;
(p) SEQ ID NOs: 8, 17, 24, 34, 38, and 46, respectively;
(q) SEQ ID NOs: 1, 17, 26, 29, 38, and 46, respectively;
(r) SEQ ID NOs: 1, 17, 26, 35, 38, and 46, respectively;
(s) SEQ ID NOs: 1, 17, 24, 33, 38, and 49, respectively;
(t) SEQ ID NOs: 9, 14, 26, 29, 38, and 46, respectively;
(u) SEQ ID NOs: 9, 14, 26, 35, 38, and 46, respectively;
(v) SEQ ID NOs: 9, 17, 24, 29, 38, and 46, respectively;
(w) SEQ ID NOs: 9, 17, 24, 35, 38, and 46, respectively;
(x) SEQ ID NOs: 9, 17, 24, 34, 38, and 46, respectively;
(y) SEQ ID NOs: 9, 14, 24, 29, 38, and 46, respectively;
(z) SEQ ID NOs: 9, 18, 26, 35, 38, and 46, respectively;
(aa) SEQ ID NOs: 8, 14, 24, 29, 38, and 46, respectively;
(bb) SEQ ID NOs: 8, 17, 26, 29, 38, and 46, respectively;
(cc) SEQ ID NOs: 9, 19, 26, 29, 38, and 46, respectively;
(dd) SEQ ID NOs: 9, 17, 26, 34, 38, and 46, respectively;
(ee) SEQ ID NOs: 10, 20, 27, 36, 42, and 50, respectively;
(ff) SEQ ID NOs: 11, 21, 28, 37, 43, and 51, respectively;
(gg) SEQ ID NOs: 12, 22, 26, 33, 38, and 46, respectively;
(hh) SEQ ID NOs: 12, 17, 26, 33, 38, and 46, respectively; and
(ii) SEQ ID NOs: 9, 17, 26, 33, 38, and 46, respectively.
|