| CPC C07K 16/3007 (2013.01) [A61K 47/10 (2013.01); A61K 47/26 (2013.01); A61K 51/0446 (2013.01); A61K 51/1051 (2013.01); A61K 51/1093 (2013.01); C07K 16/3092 (2013.01); C07K 2317/55 (2013.01); C07K 2317/71 (2013.01)] | 14 Claims |
|
1. A pharmaceutical composition comprising a labeling moiety-anti-human antibody Fab fragment conjugate, a buffering agent, a stabilizer and a nonionic surfactant and having a pH of 6.7, wherein
the anti-human antibody Fab fragment is one or more selected from the group consisting of the following (a) and (b):
(a) an anti-human CEACAM5 antibody Fab fragment comprising a heavy chain fragment consisting of the amino acid sequence represented by SEQ ID NO: 2 and a light chain consisting of the amino acid sequence represented by SEQ ID NO: 4; and
(b) an anti-human CEACAM5 antibody Fab fragment comprising a heavy chain fragment derived from a heavy chain fragment consisting of the amino acid sequence represented by SEQ ID NO: 2 by the modification of glutamic acid at amino acid position 1 of SEQ ID NO: 2 into pyroglutamic acid, and a light chain consisting of the amino acid sequence represented by SEQ ID NO: 4, and
the labeling moiety is a group represented by the following formula (I):
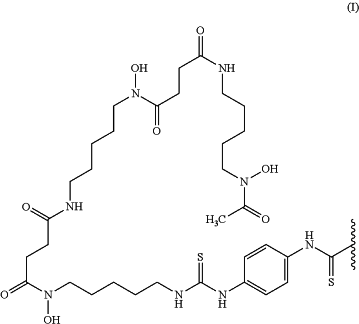 wherein the wavy line represents binding to the anti-human CEACAM5 antibody Fab fragment, where the anti-human CEACAM5 antibody Fab fragment is bound to the carbon atom of a labeling moiety terminal C(═S) group via an amino group in the anti-human CEACAM5 antibody Fab fragment,
the concentration of the labeling moiety-anti-human antibody Fab fragment conjugate is 10 mg/mL,
the nonionic surfactant comprises Polysorbate 80,
the concentration of the nonionic surfactant is 0.05 w/v %,
the buffering agent comprises citric acid,
the concentration of the buffering agent is 20 mmol/L,
the stabilizer comprises sucrose or glycerin, and
the concentration of the stabilizer is 10 to 30 w/v %.
|