| CPC C07H 1/04 (2013.01) [C07H 21/04 (2013.01)] | 13 Claims |
|
1. A method for preparing 3′-O-amino-2′-deoxyribonucleoside-5′-triphosphate, said method comprising the steps of:
(a) protecting 5′-hydroxy group of a 2′-deoxyribonucleoside;
(b) converting (S)-3′-hydroxy group of the compound obtained in step (a) into (R)-3′-hydroxy group;
(c) grafting the compound obtained in step (b) on a solid support functionalized with at least one N-hydroxyphthalimide moiety, wherein said at least one N-hydroxyphthalimide moiety is represented by formula (I):
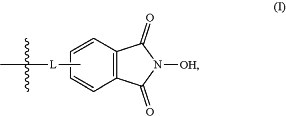 wherein L represents a linker, under conditions allowing (R)-3′-hydroxy group of the compound obtained in step (b) to be substituted by a N-hydroxyphthalimide moiety of the functionalized solid support;
(d) deprotecting 5′-hydroxy group of the compound obtained in step (c);
(e) triphosphorylating 5′-hydroxy group of the compound obtained in step (d);
(f) reacting the compound obtained in step (e) with a cleaving reagent selected from the group consisting of primary amines, hydrazine and hydroxides; and,
(g) optionally recovering 3′-O-amino-2′-deoxyribonucleoside-5′-triphosphate obtained in step (f).
|