| CPC C07F 9/5728 (2013.01) [A61K 9/0014 (2013.01); A61K 9/0019 (2013.01); A61K 9/0048 (2013.01); A61K 9/0053 (2013.01); A61K 31/404 (2013.01); A61K 31/4045 (2013.01); A61K 31/407 (2013.01); A61K 31/4162 (2013.01); A61K 31/4178 (2013.01); A61K 31/4184 (2013.01); A61K 31/4188 (2013.01); A61K 31/4192 (2013.01); A61K 31/437 (2013.01); A61K 31/4439 (2013.01); A61K 31/444 (2013.01); A61K 31/4709 (2013.01); A61K 31/496 (2013.01); A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61K 31/519 (2013.01); A61K 31/549 (2013.01); A61K 31/55 (2013.01); A61K 31/675 (2013.01); A61K 31/683 (2013.01); C07B 59/002 (2013.01); C07D 209/12 (2013.01); C07D 209/14 (2013.01); C07D 209/30 (2013.01); C07D 209/40 (2013.01); C07D 209/42 (2013.01); C07D 209/44 (2013.01); C07D 209/88 (2013.01); C07D 401/14 (2013.01); C07D 403/06 (2013.01); C07D 403/08 (2013.01); C07D 403/12 (2013.01); C07D 403/14 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 413/06 (2013.01); C07D 413/14 (2013.01); C07D 417/06 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 471/08 (2013.01); C07D 487/04 (2013.01); C07D 487/14 (2013.01); C07D 491/113 (2013.01); C07D 495/04 (2013.01); C07D 513/04 (2013.01); C07F 5/025 (2013.01); C07F 5/027 (2013.01); C07F 7/0812 (2013.01); C07F 9/65583 (2013.01); C07F 9/6561 (2013.01); C07F 9/65616 (2013.01); C07B 2200/05 (2013.01)] | 20 Claims |
|
1. A compound of Formula I
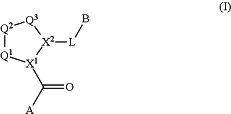 or a pharmaceutically acceptable salt thereof, wherein:
Q1 is C(R1R1′);
Q2 is C(R2R2′);
Q3 is C(R3R3′);
X1 is N and X2 is CH;
R1, R1′, R2, R2′, R3, and R3′ are independently chosen from hydrogen, halogen, hydroxyl, nitro, cyano, amino, C1-C6alkyl, C2-C6alkenyl, C1-C6alkoxyl, C2-C6alkynyl, C2-C6alkanoyl, C1-C6thioalkyl, —C(O)OR9, —OC(O)R9, —NR9C(O)R10, —C(O)NR9R10, —OC(O)NR9R10, —NR9C(O)OR10, C1-C2haloalkyl, and C1-C2haloalkoxy;
R9 and R10 are independently chosen at each occurrence from hydrogen, C1-C6alkyl, (C3-C7cycloalkyl)C0-C4alkyl, —C0-C4alkyl(C3-C7cycloalkyl), and —O—C0-C4alkyl(C3-C7cycloalkyl);
A is a group selected from:
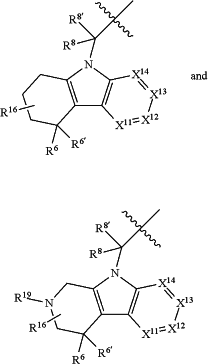 R6 is selected from —CHO, —C(O)NH2, —C(O)NH(CH3), C2-C6alkanoyl, hydrogen, hydroxyl, halogen, cyano, nitro, —COOH, —SO2NH2, vinyl, C1-C6alkyl, C2-C6alkenyl, C1-C6alkoxy, —C0-C4alkyl(C3-C7cycloalkyl), —C(O)C0-C4alkyl(C3-C7cycloalkyl), —P(O)(OR9)2, —OC(O)R9, —C(O)OR9, —C(O)N(CH2CH2R9)(R10), —NR9C(O)R10, phenyl, and 5- to 6-membered heteroaryl;
R6′ is hydrogen, halogen, hydroxyl, C1-C4alkyl, —C0-C4alkyl(C3-C7cycloalkyl), or C1-C4alkoxy; or R6 and R6′ may be taken together to form an oxo, vinyl, or imino group;
R7 is hydrogen, C1-C6alkyl, or —C0-C4alkyl(C3-C7cycloalkyl);
R8 and R8′ are independently chosen from hydrogen, halogen, hydroxyl, C1-C6alkyl, —C0-C4alkyl(C3-C7cycloalkyl), C1-C6alkoxy, and (C1-C4alkylamino)C0-C2alkyl; or R8 and R8′ are taken together to form an oxo group; or R8 and R8′ can be taken together with the carbon that they are bonded to form a 3-membered carbocyclic ring;
X11 is N or CR11;
X12 is CR12;
X13 is CR13;
X14 is N or CR14;
one of R12 and R13 is chosen from R31 and the other of R12 and R13 is chosen from R32:
R31 is chosen from hydrogen, halogen, hydroxyl, nitro, cyano, amino, —COOH, C1-C2haloalkyl, C1-C2haloalkoxy, C1-C6alkyl, —C0-C4alkyl(C3-C7cycloalkyl), C2-C6alkenyl, C2-C6alkanoyl, C1-C6alkoxy, C2-C6alkenyloxy, —C(O)OR9, C1-C6thioalkyl, —C0-C4alkylNR9R10, —C(O)NR9R10, —SO2R9, —SO2NR9R10, —OC(O)R9, and —C(NR9)NR9R10;
R32 is 5-6 membered heteroaryl having 1, 2, or 3 heteroatoms independently chosen from N, O, and S wherein the heteroaryl ring can be optionally substituted with one or more substituents independently chosen from halogen, hydroxyl, amino, cyano, —CHO, —COOH, —CONH2, C1-C6alkyl, C2-C6alkenyl, C2-C6alkynyl, —C1-C6alkoxy, C2-C6alkanoyl, C1-C6alkylester, (mono- and di-C1-C6alkylamino)C0-C2alkyl, C1-C2haloalkyl, hydoxyC1-C6alkyl, ester, carbamate, urea, sulfonamide, —C1-C6alkyl(heterocyclo), C1-C6alkyl(heteroaryl), —C1-C6alkyl(C3-C7cycloalkyl), O—C1-C6alkyl(C3-C7cycloalkyl), and C1-C2haloalkoxy;
R11 and R14 are independently chosen at each occurrence from hydrogen, halogen, hydroxyl, nitro, cyano, —O(PO)(OR9)2, —(PO)(OR9)2, C1-C6alkyl, C2-C6alkenyl, C2-C6alkynyl, C2-C6alkanoyl, C1-C6alkoxy, C1-C6thioalkyl, —C0-C4alkyl(mono- and di-C1-C6alkylamino), —C0-C4alkyl(C3-C7cycloalkyl), —C0-C4alkoxy(C3-C7cycloalkyl), C1-C2haloalkyl, and C1-C2haloalkoxy;
R16 is absent or may include one or more substituents independently chosen from halogen, hydroxyl, nitro, cyano, C1-C6alkyl, C2-C6alkenyl, C2-C6alkanoyl, C1-C6alkoxy, —C0-C4alkyl(mono- and di-C1-C6alkylamino), —C0-C4alkyl(C3-C7cycloalkyl), C1-C2haloalkyl, and C1-C2haloalkoxy;
R19 is hydrogen, C1-C6alkyl, C2-C6alkenyl, C2-C6alkanoyl, —SO2C1-C6alkyl, (mono- and di-C1-C6alkylamino)C1-C4alkyl, —C0-C4alkyl(C3-C7cycloalkyl), —C0-C4alkyl(C3-C7heterocycloalkyl), —C0-C4alkyl(aryl), C0-C4alkyl(heteroaryl) and wherein R19 other than hydrogen is unsubstituted or substituted with one or more substituents independently chosen from halogen, hydroxyl, amino, —COOH, and —C(O)OC1-C4alkyl;
R21 and R22 are independently chosen at each occurrence from hydrogen, hydroxyl, cyano, amino, C1-C6alkyl, C1-C6haloalkyl, C1-C6alkoxy, (C3-C7cycloalkyl)C0-C4alkyl, (phenyl)C0-C4alkyl, —C1-C4alkylOC(O)OC1-C6alkyl, —C1-C4alkylOC(O)C1-C6alkyl, —C1-C4alkylC(O)OC1-C6alkyl, (4- to 7-membered heterocycloalkyl)C0-C4alkyl having 1, 2, or 3 heteroatoms independently chosen from N, O, and S, and (5- or 6-membered unsaturated or aromatic heterocycle)C0-C4alkyl having 1, 2, or 3 heteroatoms independently chosen from N, O, and S;
R23 is independently chosen at each occurrence from C1-C6alkyl, C1-C6haloalkyl, (aryl)C0-C4alkyl, (C3-C17cycloalkyl)C0-C4alkyl, (phenyl)C0-C4alkyl, (4- to 7-membered heterocycloalkyl)C0-C4alkyl having 1, 2, or 3 heteroatoms independently chosen from N, O, and S, and (5- or 6-membered unsaturated or aromatic heterocycle)C0-C4alkyl having 1, 2, or 3 heteroatoms independently chosen from N, O, and S;
L is
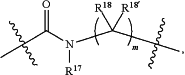 where R17 is hydrogen, C1-C6alkyl, or —C0-C4alkyl(C3-C7cycloalkyl), and R18 and R18′ are independently chosen from hydrogen, halogen, hydroxymethyl, and methyl; and m is 0, 1, 2, or 3;
B is a monocyclic or bicyclic carbocyclic; a monocyclic or bicyclic carbocyclic-oxy group; a monocyclic, bicyclic, or tricyclic heterocyclic group having 1, 2, 3, or 4 heteroatoms independently selected from N, O, and S and from 4 to 7 ring atoms per ring; C2-C6alkenyl; C2-C6alkynyl; —(C0-C4alkyl)(aryl); or —(C0-C4alkyl)(heteroaryl); each of which B is unsubstituted or substituted with one or more substituents independently chosen from R33 and R34, and 0 or 1 substituents chosen from R35 and R36;
R33 is independently chosen from halogen, hydroxyl, —COOH, cyano, C1-C6alkyl, C2-C6alkanoyl, C1-C6alkoxy, —C0-C4alkylNR9R10, —SO2R9, C1-C2haloalkyl, and C1-C2haloalkoxy;
R34 is independently chosen from nitro, C2-C6alkenyl, C2-C6alkynyl, C1-C6thioalkyl, -JC3-C7cycloakyl, —B(OH)2, -JC(O)NR9R23, -JOSO2OR21, —C(O)(CH2)1-4S(O)R21, —O(CH2)1-4S(O)NR21R22, -JOP(O)(OR21)(OR22), -JP(O)(OR21)(OR22), -JOP(O)(OR21)R22, -JP(O)(OR21)R22, -JOP(O)R21R22, -JP(O)R21R22, -JSP(O)(OR21)(OR22), -JSP(O)(OR21)(R22), -JSP(O)(R21)(R22), -JNR9P(O)(NHR21)(NHR22), -JNR9P(O)(OR21)(NHR22), -JNR9P(O)(OR21)(OR22), -JC(S)R21, -JNR21SO2R22, -JNR9S(O)NR10R22, -JNR9SO2NR10R22, -JSO2NR9COR22, -JSO2NR9CONR21R22, -JNR21SO2R22, -JC(O)NR21SO2R22, -JC(NH2)NR22, -JOC(O)NR21R22, -JNR21C(O)OR22, -JNR21OC(O)R22, —(CH2)1-4C(O)NR21R22, -JNR9C(O)R21, -JC(O)R21, -JNR9C(O)NR10R22, —CCR21, —(CH2)1-4OC(O)R21, and -JC(O)OR23; each of which R34 may be unsubstituted or substituted with one or more substituents independently chosen from halogen, hydroxyl, nitro, cyano, amino, oxo, —B(OH)2, —Si(CH3)3, —COOH, —CONH2, —P(O)(OH)2, C1-C6alkyl, —C0-C4alkyl(C3-C7cycloalkyl), C1-C6alkoxy, —C0-C2alkyl(mono- and di-C1-C4alkylamino), C1-C6alkylester, C1-C4alkylamino, C1-C4hydroxylalkyl, C1-C2haloalkyl, and C1-C2haloalkoxy,
R35 is independently chosen from naphthyl, naphthyloxy, indanyl, (4- to 7-membered heterocycloalkyl)C0-C4alkyl containing 1 or 2 heteroatoms chosen from N, O, and S, and bicyclic heterocycle containing 1, 2, or 3 heteroatoms independently chosen from N, O, and S, and containing 4- to 7-ring atoms in each ring; each of which R35 is unsubstituted or substituted with one or more substituents independently chosen from halogen, hydroxyl, nitro, cyano, C1-C6alkyl, C2-C6alkenyl, C2-C6alkanoyl, C1-C6alkoxy, (mono- and di-C1-C6alkylamino)C0-C4alkyl, C1-C6alkylester, —C0-C4alkyl(C3-C7cycloalkyl), —SO2R9, C1-C2haloalkyl, and C1-C2haloalkoxy; and
R36 is independently chosen from tetrazolyl, (phenyl)C1-C2alkoxy, phenoxy, and 5- or 6-membered heteroaryl containing 1, 2, or 3 heteroatoms independently chosen from N, O, B, and S, each of which R36 is unsubstituted or substituted with one or more substituents independently chosen from halogen, hydroxyl, nitro, cyano, C1-C6alkyl, C2-C6alkenyl, C2-C6alkanoyl, C1-C6alkoxy, (mono- and di-C1-C6alkylamino)C0-C4alkyl, C1-C6alkylester, —C0-C4alkyl(C3-C7cycloalkyl), —SO2R9, —OSi(CH3)2C(CH3)3, —Si(CH3)2C(CH3)3, C1-C2haloalkyl, and C1-C2haloalkoxy; and
J is independently selected at each occurrence from a covalent bond, C1-C4alkylene, —OC1-C4alkylene, C2-C4alkenylene, and C2-C4alkynylene.
|