| CPC C07D 513/04 (2013.01) [A61P 37/06 (2018.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01); C07D 487/04 (2013.01)] | 9 Claims |
|
1. A compound of formula I, or a pharmaceutically acceptable salt, stereoisomer, enantiomer, diastereomer, atropisomer, optical isomer, racemate, polymorph, solvate or isotopically labeled compound thereof:
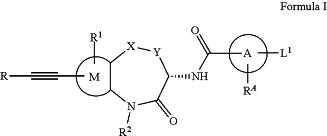 wherein
X is S, SO,
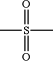 Y is C1-C2 alkylene;
Ring A is a benzene ring, a 5-6 membered heteroaromatic ring, a 5-6 membered non-aromatic heterocyclic ring or
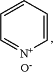 and the carbonyl moiety and L1 connected to Ring A are in meta-positions;
RA is H or C1-C4 alkyl;
L1 is C3-C6 alkyl, C3-C6 alkoxy, halogenated C3-C6 alkoxy, C3-C6 alkenyl, C3-C6 alkenyloxy, or
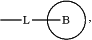 wherein
L is O, S, NH, N(CH3), CH2, CH2CH2, CH(CH3), CHF, CF2, CH2O, CH2N(CH3), CH2NH or CH(OH);
Ring B is a C3-C6 cycloalkyl, phenyl, 5-6 membered heteroaryl or 5-6 membered non-aromatic heterocyclyl, wherein the C3-C6 cycloalkyl, the phenyl, the 5-6 membered heteroaryl and the 5-6 membered non-aromatic heterocyclyl are each independently unsubstituted or substituted with one or two substituents each independently selected from the group consisting of halogen, C1-C4 alkyl, halogenated C1-C4 alkyl, C1-C4 alkoxy, halogenated C1-C4 alkoxy, nitro and C1-C4 alkyl C(O)—;
R2 is H or CH3;
Ring M is independently a C6-C10 aromatic ring or a 5-10 membered heteroaromatic ring;
R1 representative 1-3 substituents each independently being H, halogen, —OH, —CN, —COOH,
Ct-Co alkyl, C1-C6 haloalkyl, C1-C6 alkoxy, C1-C6 haloalkoxy, C2-C10 alkoxyalkyl, C2-C10 haloalkoxyalkyl, C1-C6 hydroxyalkyl, —B(OH)2, —S(O)n1Ra, —N(Ra)2, —C(═O)N(Ra)2, —NHC(═O)Ra, —NHC(═O)ORa, —NHC(═O)C(═O)N(R1)2, —NHC(═O)C(═O)ORa, —NHC(═O)N(Ra)2, —NHC(═O)NRaC(═O)N(Ra)2, —NHC(═O)NRaS(O)2ORa, —NHC(═O)NRaS(O)2N(Ra)2, —NHC(═S)N(Ra)2, —NHC(═N—C≡N)NRa, —NHC(═N—C≡N)SRa, —NHS(O)n1Ra, Ma, —(C1-C6 alkylene)-B(OH)2, —(C1-C6 alkylene)-S(O)n1Ra, —(C1-C6 alkylene)-N(Ra)2, —(C1-C6 alkylene)-C(═O)N(Ra)2, —(C1-C6 alkylene)-NHC(═O)Ra, —(C1-C6 alkylene)-NHC(═O)ORa, —(C1-C6 alkylene)-NHC(═O)C(═O)N(Ra)2, —(C1-C6 alkylene)-NHC(═O)C(═O)ORa, —(C1-C6 alkylene)-NHC(═O)N(Ra)2, —(C1-C6 alkylene)-NHC(═O)NRaC(═O)N(Ra)2, —(C1-C6 alkylene)-NHC(═O)NRAS(O)2ORa, —(C1-C6 alkylene)-NHC(═O)NRaS(O)2N(Ra)2, —(C1-C6 alkylene)-NHC(═S)N(Ra)2, —(C1-C6 alkylene)-NHC(═N—C≡N)NRa, —(C1-C6 alkylene)-NHC(═N—C≡N)SRa, —(C1-C6 alkylene)-NHS(O)n1Ra, —(C1-C6 alkylene)-Ma, —OMa, —SMa, or —N(Ra)Ma;
Ra at each occurrence is independently hydrogen, C1-C6 alkyl, C6-C10 aryl, 5-10 membered heteroaryl, 3-10 membered non-aromatic heterocyclyl, C3-C10 cycloalkyl, or C5-C10 cycloalkenyl, wherein the C1-C6 alkyl, the C6-C10 aryl, the 5-10 membered heteroaryl, the 3-10 membered non-aromatic heterocyclyl, the C3-C10 cycloalkyl and the C5-C10 cycloalkenyl are each independently unsubstituted or substituted with one or two selected from the group consisting of amino, hydroxyl, C1-C4 alkoxy, C1-C6 alkyl, C3-C10 cycloalkyl, and CN;
Ma at each occurrence is independently C6-C10 aryl, 5-10 membered heteroaryl, 3-10 membered non-aromatic heterocyclyl, C3-C10 cycloalkyl, or C3-C10 cycloalkenyl, wherein the C6-C10 aryl, the 5-10 membered heteroaryl, the 3-10 membered non-aromatic heterocyclyl, the C3-C10 cycloalkyl, and the C3-C10 cycloalkenyl are each independently unsubstituted or substituted with one or two selected from the group consisting of halogen, C1-C4 alkyl, C1-C4 alkoxy, and —CN;
n1 at each occurrence is independently 0, 1 or 2;
R is each independently hydrogen, C1-C10 alkyl, C6-C10 aryl, 5-10 membered heteroaryl, 3-10 membered non-aromatic heterocyclyl, C3-C10 cycloalkyl, or C3-C10 cycloalkenyl, wherein the C1-C10 alkyl, the C6-C10 aryl, the 5-10 membered heteroaryl, the 3-10 membered non-aromatic heterocyclyl, the C3-C10 cycloalkyl, and the C3-C10 cycloalkenyl are each independently unsubstituted or substituted with 1 to 4 Mds;
Md at each occurrence is independently C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, halogen, C1-C6 haloalkyl, —CN, NO2, SCF3, oxo, —OMe, —OC(O)Mh, —OC(O)NMfMg, —SMe, —S(O)2Me, —S(O)2NMfMg, —C(O)Me, —C(O)-(5-10 membered monocyclic heterocyclic ring), —C(O)-(5-10-membered monocyclic heteroaryl), —C(O)OMe, —C(O)NMfMg, —NMfMg, —N(Me)C(O)Mh, —N(Me)S(O)2M, —N(M)C(O)OMh, —N(M)C(O)NMfMg, —(C1-C6 alkylene)-OMe, —(C1-C6 alkylene)-OC(O)Mh, —(C1-C6 alkylene)-OC(O)NMfMg, —(C1-C6 alkylene)-S(O)2Me, —(C1-C6 alkylene)-S(O)2NMfM9, —(C1-C6 alkylene)-C(O)Me, —(C1-C6 alkylene)-C(O)OMh, —(C1-C6 alkylene)-C(O)NMfMg, —(C1-C6 alkylene)-NMfMg, —(C1-C6 alkylene)-N(Me)C(O)M, —(C1-C6 alkylene)-N(Me)S(O)2Mh, —(C1-C6 alkylene)-N(Me)C(O)OMh, —(C1-C6 alkylene)-N(Me)C(O)NMfMg, —(C1-C6 alkylene)-CN, C6-C10 aryl, 5-10 membered heteroaryl, 3-10 membered non-aromatic heterocyclyl, C3-C10 cycloalkyl, or C3-C10 cycloalkenyl, wherein the C6-C10 aryl, the 5-10 membered heteroaryl, the 3-10 membered non-aromatic heterocyclyl, the C3-C10 cycloalkyl and the C3-C10 cycloalkenyl are each independently unsubstituted or substituted by one or two substituents each independently selected from the group consisting of halogen, C1-C4 alkyl, C1-C4 alkoxy, and —CN;
Me, Mf, Mg and Mh at each occurrence are each independently hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, C3-C10 cycloalkyl, C6-C10 aryl, 5-10 membered heteroaryl, or 3-10 membered non-aromatic heterocyclyl, wherein the Ct-Co alkyl, the C1-C10 cycloalkyl, the C6-C10 aryl, the 5-10 membered heteroaryl, and the 3-10 membered non-aromatic heterocyclyl are each independently unsubstituted or substituted by one or two substituents each independently selected from the group consisting of halogen, hydroxy, C1-C4 alkyl, C1-C4 alkoxy, —CN, —S(O)2(C1-C4 alkyl), and —C(O)(C1-C4 alkyl); or
two Mds together with the ring atoms to which they are connected, form a 3-8 membered saturated or unsaturated ring.
|