| CPC C07D 487/04 (2013.01) [A61K 31/4184 (2013.01); C07D 401/04 (2013.01); C07D 403/04 (2013.01); C07D 403/14 (2013.01); C07D 413/04 (2013.01); C07D 471/04 (2013.01); C07D 491/10 (2013.01); C07D 495/10 (2013.01)] | 29 Claims |
|
1. A method of treating ovarian, breast, prostate, or lung cancer, comprising administering to a subject in need thereof an effective amount of a compound according to Formula I:
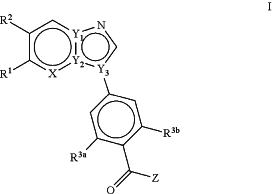 wherein,
X is N or CR4;
one of Y1, Y2 and Y3 is N and the other two are C;
Z is
—NR5aR5b,
—NR5c—, wherein the N atom and R3b together with the atoms onto which they are attached form a fused 5-6 membered heterocycloalkenyl comprising one double bond and further comprising zero, one, or two additional heteroatoms independently selected from N, O, and S, or
N-linked 4-7 membered heterocycloalkyl further comprising zero, one, or two additional heteroatoms independently selected from N, O, and S, optionally substituted with one, two or three independently selected R6 groups;
R1 is H, halo, C1-4 alkyl, or C1-4 alkoxy optionally substituted with C1-4 alkoxy, phenyl, —CN, —C(═O)OH, or —C(═O)—C1-4 alkoxy;
R2 is 5-6 membered monocyclic heteroaryl comprising one, two or three heteroatoms independently selected from N, O, and S, which heteroaryl is optionally substituted with one or more independently selected R7 groups;
R3a and R3b are independently selected from
halo,
C1-4 alkyl,
C1-4 alkoxy optionally substituted with one or more independently selected halo, —OH or C1-4 alkoxy,
NR5aR5b, and
—OH;
R4 is H or C1-4 alkyl;
R5a is H or C1-4 alkyl;
R5b is selected from
C1-6 alkyl optionally substituted with one or more independently selected R9,
C3-7 cycloalkyl optionally substituted with one or more independently selected R10,
4-7 membered monocyclic heterocycloalkyl comprising one, two or three heteroatoms independently selected from N, O, and S, which heterocycloalkyl is optionally substituted with one or more oxo, and
5-6 membered monocyclic heteroaryl comprising one, two or three heteroatoms independently selected from N, O, and S, which heteroaryl is optionally substituted with one or more independently selected C1-4 alkyl;
R5c is selected from C3-7 cycloalkyl, and C1-6 alkyl optionally substituted with one or more independently selected halo;
each R6 is independently selected from
oxo,
halo,
—CN,
—OH,
—NR11aR11b
phenyl,
C3-7 cycloalkyl,
C2-4 alkynyl,
—C(═O)—C1-4 alkoxy,
C1-4 alkoxy optionally substituted with one or more halo or phenyl,
C1-4 alkyl optionally substituted with one or more halo, —OH, or C1-4 alkoxy, and
4-7 membered monocyclic heterocycloalkyl comprising one, two or three heteroatoms independently selected from N, O, and S;
each R7 is selected from
halo,
—CN,
C1-6 alkyl optionally substituted with one or more independently selected
halo,
—CN,
—OH,
C1-4 alkoxy optionally substituted with one or more independently selected halo,
—NR11cR11d,
—C(═O)R2, or
4-6 membered monocyclic heterocycloalkyl comprising one, two or three heteroatoms independently selected from N, O, and S,
C1-4 alkoxy,
C3-7 cycloalkyl,
4-6 membered monocyclic heterocycloalkyl comprising one, two or three heteroatoms independently selected from N, O, and S, which heterocycloalkyl is optionally substituted with —C(═O)C1-4 alkoxy or C1-4 alkyl optionally substituted with —CN,
—NR13aR13b, and
—C(═O)NR13cR13d;
each R8a and R8b is independently selected from H and C1-4 alkyl optionally substituted with one —OH or C1-4 alkoxy;
each R9 is independently selected from
halo,
—CN,
—NR11eR11f
—OH,
C1-4 alkoxy,
—S(═O)2—C1-4 alkyl,
4-7 membered monocyclic heterocycloalkyl comprising one, two or three heteroatoms independently selected from N, O, and S, and
5-6 membered monocyclic heteroaryl comprising one, two or three heteroatoms independently selected from N, O, and S, which heteroaryl is optionally substituted with one or more independently selected C1-4 alkyl;
each R10 is independently selected from
halo,
C1-4 alkyl optionally substituted with one or more independently selected halo, —OH, or C1-4 alkoxy,
—OH,
C1-4 alkoxy, and
—NR11gR11h;
each R11a, R11b, R11c, R11d, R11e, R11f, R11g, and R11h is independently selected from H and C1-4 alkyl; each R12 is
—NR14aR14b, wherein each R14a and R14b is independently selected from H and C1-4 alkyl,
—OH,
C1-4 alkoxy optionally substituted with one or more independently selected C3-7 cycloalkyl, halo, —NR15aR15b or 4-6 membered monocyclic heterocycloalkyl comprising one, two or three heteroatoms independently selected from N, O, and S,
—O-(4-6 membered monocyclic heterocycloalkyl comprising one, two or three heteroatoms independently selected from N, O, and S), or
—O—(C3-7 monocyclic cycloalkyl);
each R13a, R13b, R13c, and R13d, is independently selected from H and C1-4 alkyl; and
each R15a and R15b is independently selected from H and C1-4 alkyl;
or a pharmaceutically acceptable salt thereof.
|