| CPC C07D 471/04 (2013.01) [A61P 35/00 (2018.01)] | 33 Claims |
|
1. A method of treating a MEK-inhibitor responsive disorder, MEK-inhibitor responsive dermal disorder, MEK-mediated disorder, or a MEK-mediated dermal disorder comprising administering to a subject in need thereof, a therapeutically effective amount of a compound of Formula (I):
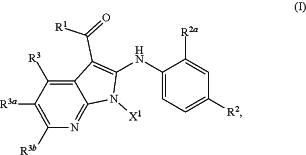 or an N-oxide, a stereoisomer, mixture of stereoisomers, and/or a pharmaceutically acceptable salt thereof,
wherein:
R1 is —OR4, —NR5R5a, or an N-linked heterocycloalkyl wherein the N-linked heterocycloalkyl is optionally substituted with one or two R10;
R2a is halo or C1-C6 alkyl;
R2 is —S—C1-C6 alkyl, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, or halo;
X1 is C1-C6 alkyl;
R3, R3a, and R3b are independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, —S—C1-C6 alkyl, halo, C1-C6 alkoxy, C3-C8-cycloalkyloxy, heterocycloalkyloxy, heteroaryloxy, or phenoxy wherein each phenyl and heteroaryl is independently optionally substituted with 1, 2, or 3 R6;
R4 is C1-C6 alkyl, C3-C8 cycloalkyl, C3-C8 cycloalkylalkyl, C1-C6 hydroxyalkyl, or C1-C6 alkoxyalkyl;
R5 is hydrogen, C1-C6 alkyl optionally substituted with one heterocycloalkyl, C3-C8 cycloalkyl, C3-C8 cycloalkyl-C1-C6-alkyl-, C1-C6 hydroxyalkyl, C1-C6 alkoxy-C1-C6-alkyl, or —OR5b;
R5a is hydrogen or C1-C6 alkyl;
R5b is hydrogen, C1-C6 alkyl, C3-C8 cycloalkyl, C3-C8 cycloalkyl-C1-C6-alkyl, C1-C6 hydroxyalkyl, or C1-C6 alkoxy-C1-C6-alkyl;
each R6 is independently selected from the group consisting of carboxy, C1-C6-alkoxycarbonyl, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy-C1-C6-alkyl, C3-C8 cycloalkyl, heterocycloalkyl, —OC(O)R7, —OS(O)2R7, —O—C1-C6-haloalkyl, C3-C8 cycloalkyloxy, heterocycloalkyloxy, aryloxy, heteroaryloxy, amino, C1-C6-alkylamino, di-C1-C6-alkylamino, —NR8aS(O)2R8, —NR8aC(O)R8, —S(O)2R8, —S(O)2NR8aR8, —C(O)R8, —C(O)NR8aR8, and —C1-C6-alkylene-R6a;
each R6a is independently selected from the group consisting of C3-C8 cycloalkyl, heterocycloalkyl, —NH2, —NH(C1-C6-alkyl), —N(C1-C6-alkyl)2, —NR9aS(O)2R9, —NR9aC(O)R9, —S(O)2R9, —S(O)2NR9aR9, —C(O)R9, and —C(O)NR9aR9;
each R7 is independently selected from the group consisting of amino, C1-C6-alkylamino, di-C1-C6-alkylamino, C1-C6 alkyl, C3-C8 cycloalkyl, C1-C6-alkoxy, heterocycloalkyl, aryl, and heteroaryl;
each R8a and R9a is independently H or C1-C6 alkyl;
each R8 and R9 is independently C1-C6 alkyl, aryl, heteroaryl, C3-C8 cycloalkyl, or heterocycloalkyl; and
each R10 is independently hydrogen, halo, hydroxy, oxo, C1-C6 alkyl, C1-C6 hydroxyalkyl, C1-C6 haloalkyl, amino-C1-C6-alkyl, C1-C6-alkylamino-C1-C6-alkyl, di-C1-C6-alkylamino-C1-C6-alkyl, C1-C6-alkoxy, C3-C8 cycloalkyl, heterocycloalkyl, or heteroaryl.
|