| CPC C07D 471/04 (2013.01) [C07D 209/94 (2013.01); C07D 215/14 (2013.01); C07D 401/04 (2013.01); C07D 403/04 (2013.01)] | 19 Claims |
|
1. A compound of formula (I), or a pharmaceutically acceptable salt thereof:
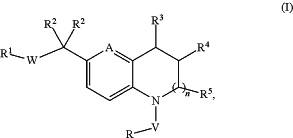 wherein:
n is 0 or 1;
A is selected from (1) —CH═ and (2) —N═;
V is selected from (1) absent and (2) —C(O)—;
W is selected from (1) —C(O)—NH— and (2) —NH—C(O)—;
R1 is selected from (1) aryl and (2) heteroaryl, wherein each of the aryl and heteroaryl is optionally substituted with 1-3 substituents independently selected from:
(a) halogen, and
(b) C1-6 alkyl, optionally substituted with 1-4 halogens;
each occurrence of R2 is independently selected from: (1) hydrogen and (2) C1-6 alkyl;
or alternatively, the two R2 groups together with the carbon to which they are attached form a C3-4 cycloalkyl, wherein the C3-4 cycloalkyl is optionally substituted with C1-6 alkyl;
R3 is hydrogen, n is 1, and R4 and R5 together with the carbons to which they are attached form a C3-4 cycloalkyl;
or alternatively, R5 is hydrogen, n is 1, and R3 and R4 together with the carbons to which they are attached form a C3-4 cycloalkyl;
or alternatively, R5 is absent, n is 0, and R3 and R4 together with the carbons to which they are attached form a C3-4 cycloalkyl;
or alternatively, R4 is selected from (1) hydrogen and (2) C1-6 alkyl optionally substituted with 1-3 halogens, n is 1, and R3 and R5 together with the carbons to which they are attached and the carbon to which R4 is attached form a C4-5 cycloalkyl;
R is selected from:
(1) hydrogen,
(2) aryl,
(3) C1-6 alkyl, optionally substituted with 1-4 substituents halogens,
(4) C3-6 cycloalkyl,
(5) —O—C1-6 alkyl, optionally substituted with phenyl,
(6) —O—C3-6 cycloalkyl,
(7) —O-heterocyclyl, and
(8) heteroaryl,
wherein each of the aryl of (2) and heteroaryl of (8) is optionally substituted with 1-3 substituents independently selected from:
(a) halogen,
(b) —OH,
(c) C1-6 alkyl, optionally substituted with 1-4 substituents independently selected from (i) —OH and (ii) halogen,
(d) —O—C1-6 alkyl, optionally substituted with 1-4 substituents independently selected from (i) —OH and (ii) halogen,
(e) C3-6 cycloalkyl,
(f) —O—C3-6 cycloalkyl, and
(g) —C(O)—O—C1-6 alkyl.
|