| CPC C07D 401/14 (2013.01) [C07B 2200/13 (2013.01)] | 12 Claims |
|
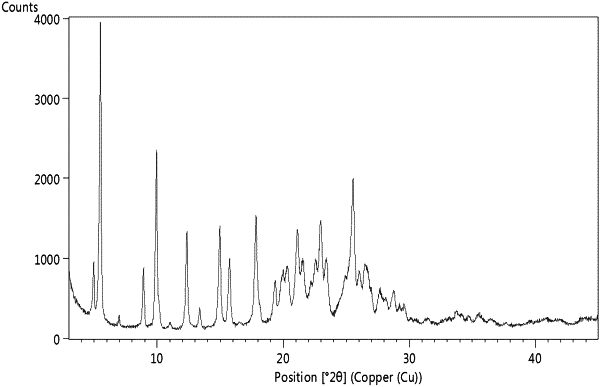
1. Crystalline nilotinib hydrochloride Form L characterized by a powder X-Ray diffraction pattern having at least six peaks selected from 4.9, 5.5, 6.9, 8.9, 9.9, 11, 13.3, 17.8, 19.9, 21.1, 21.5, 22.9, 26.6, 27.6, 28.7, and 29.5° 2θ, where all the peak values listed are ±0.2° 2θ.
|