| CPC C07D 277/34 (2013.01) [A61K 31/381 (2013.01); A61K 31/426 (2013.01); A61K 31/427 (2013.01); A61K 31/429 (2013.01); A61K 31/433 (2013.01); A61K 31/4365 (2013.01); A61K 31/437 (2013.01); A61K 31/4439 (2013.01); A61K 31/454 (2013.01); A61K 31/4709 (2013.01); A61K 31/4725 (2013.01); A61K 31/497 (2013.01); A61K 31/4985 (2013.01); A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61K 31/519 (2013.01); A61K 39/39 (2013.01); A61K 45/06 (2013.01); C07D 277/36 (2013.01); C07D 277/40 (2013.01); C07D 403/06 (2013.01); C07D 417/04 (2013.01); C07D 417/06 (2013.01); C07D 417/10 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 495/04 (2013.01); C07D 513/04 (2013.01)] | 32 Claims |
|
1. A compound, wherein the compound is one of Formula Ib:
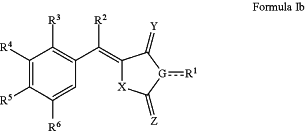 wherein:
X is S;
Y is O or S;
Z is O, or NR1a;
G is Nor C;
if G is N, and Z is O, then R1 is methyl;
if G is N, and Z is NR1a, then R1-R1a are connected as a —CH2CH2—, —CH2CH2CH2—, —CH═CH—, —C(CH3)═CH— or —CH═C(CH3)— group; and
if G is C, then Z is NR1a and R1-R1a are connected as a ═CH—CH═CH—, ═N—CH═CH—, or ═CH—N═CH— group;
R2 is hydrogen, halogen, C1-6alkyl, or C1-6alkyl substituted with one or more halogen, thiol, hydroxyl, carbonyl, carboxyl, carbonyloxyl, C1-6alkoxy, C1-6hydroxyalkoxy, amino, C1-6alkylamino, di(C1-6alkyl)amino, or azido groups;
R3 is halogen or R2-R3 are connected as a —CH2CH2— or —CH2CH2CH2—group;
R5 is hydrogen, halogen, —SR3a, —S(O)R3a, —OR3a, —OCH2R3b, —OCH(CH3)R3b, —OC(O)NHR3a, —NR3aR4a, —NHSO2R3a, azido, —CHO, CO2R3a, cyano, C1-6alkyl, —CR5aR6aR7a, C2-6alkenyl, —C(R5a)═C(R8a)(R9a), C2-6alkynyl, or —C≡CR8a;
wherein R3a, R3b, and R4a are independently hydrogen, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, tetrazolyl, C1-6alkyl, cyclic —(C1-8alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups;
wherein the phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups are optionally substituted with 1-3 substituents independently selected from halogen, thiol, C1-6alkyl thioether, C1-6alkyl sulfoxide, C1-6alkyl, C1-6alkoxyl, amino, C1-6alkylamino, C1-6dialkylamino, C1-6alkyl sulfonamide, azido, —CHO, —CO2H, C1-6alkyl carboxylate, cyano, C2-6alkenyl, and C2-6alkynyl groups; and
wherein the C1-6alkyl, cyclic —(C1-8alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups are optionally substituted with one or more halogen, thiol, hydroxyl, carbonyl, carboxyl, carbonyloxyl, C1-6alkoxy, C1-6hydroxyalkoxy, amino, C1-6alkylamino, di(C1-6alkyl)amino, azido, piperidinyl, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups;
wherein R5a, R6a, R7a, R8a and R9a are independently hydrogen, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, tetrazolyl, C1-6alkyl, cyclic —(C1-8alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, C2-6alkynyl, C1-6alkoxyl, cyclic —(C1-8alkoxyl)-, cyclic —(C1-6oxaalkoxyl)-, or cyclic —(C1-6azaalkoxyl)-groups;
wherein the phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups are optionally substituted with 1-3 substituents independently selected from halogen, thiol, C1-6alkyl thioether, C1-6alkyl sulfoxide, C1-6alkyl, C1-6alkoxyl, amino, C1-6alkylamino, C1-6dialkylamino, C1-6alkyl sulfonamide, azido, —CHO, —CO2H, C1-6alkyl carboxylate, cyano, C2-6alkenyl, and C2-6alkynyl group; and
wherein the C1-6alkyl, cyclic —(C1-8alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups are optionally substituted with one or more halogen, thiol, hydroxyl, carbonyl, carboxyl, carbonyloxyl, C1-6alkoxy, C1-6hydroxyalkoxy, amino, C1-6alkylamino, di(C1-6alky)amino, azido, piperidinyl, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups;
R6 is hydrogen, halogen, —SR3a, —S(O)R3a, —OC(O)NHR3a, —NR3aR4a, —NHSO2R3a, azido, —CHO, CO2R3a, cyano, C1-6alkyl, —CR5aR6aR7a, C2-6alkenyl, —C(R5a)═C(R8a)(R9a), C2-6alkynyl, or —C≡CR8a;
wherein R3a, R3b, and R4a are independently hydrogen, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, tetrazolyl, C1-6alkyl, cyclic —(C1-8alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups;
wherein the phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups are optionally substituted with 1-3 substituents independently selected from halogen, thiol, C1-6alkyl thioether, C1-6alkyl sulfoxide, C1-6alkyl, C1-6alkoxyl, amino, C1-6alkylamino, C1-6dialkylamino, C1-6alkyl sulfonamide, azido, —CHO, —CO2H, C1-6alkyl carboxylate, cyano, C2-6alkeny, and C2-6alkynyl groups; and
wherein the C1-6alkyl, cyclic —(C1-8alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups are optionally substituted with one or more halogen, thiol, hydroxyl, carbonyl, carboxyl, carbonyloxyl, C1-6alkoxy, C1-6hydroxyalkoxy, amino, C1-6alkylamino, di(C1-6alkyl)amino, azido, piperidinyl, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups;
wherein R5a, R6a, R7a, R8a and R9a are independently hydrogen, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, tetrazolyl groups, C1-6alkyl, cyclic —(C1-6alkyl)-, cyclic —(C1-6oxaalkyl)-cyclic —(C1-6azaalkyl)-, C2-6alkenyl, C2-6alkynyl, C1-6alkoxyl, cyclic —(C1-6alkoxyl)-, cyclic —(C1-6oxaalkoxyl)-, or cyclic —(C1-6azaalkoxyl)-;
wherein the phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups are optionally substituted with 1-3 substituents independently selected from halogen, thiol, C1-6alkyl thioether, C1-6alkyl sulfoxide, C1-6alkyl, C1-6alkoxyl, amino, C1-6alkylamino, C1-6dialkylamino, C1-6alkyl sulfonamide, azido, —CHO, —CO2H, C1-6alkyl carboxylate, cyano, C2-6alkenyl, and C2-6alkynyl groups; and
wherein the C1-6alkyl, cyclic —(C1-8alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups are optionally substituted with one or more halogen, thiol, hydroxyl, carbonyl, carboxyl, carbonyloxyl, C1-6alkoxy, C1-6hydroxyalkoxy, amino, C1-6alkylamino, di(C1-6alkyl)amino, azido, piperidinyl, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups; or
R6 is —OR3a, —OCH2R3b, or —OCH(CH3)R3b;
wherein R3a and R3b are independently phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, tetrazolyl, C1-6alkyl, cyclic —(C1-6alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups;
wherein the phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups are optionally substituted with 1-3 substituents independently selected from of halogen, thiol, C1-6alkyl thioether, C1-6alkyl sulfoxide, C1-6alkyl, C1-6alkoxyl, amino, C1-6alkylamino, C1-6dialkylamino, C1-6alkyl sulfonamide, azido, —CHO, —CO2H, C1-6alkyl carboxylate, cyano, C2-6alkeny, and C2-6alkynyl groups; and
wherein the C1-6alkyl, cyclic —(C1-6alkyl)-, cyclic —(C1-6oxaalkyl)-, cyclic —(C1-6azaalkyl)-, C2-6alkenyl, or C2-6alkynyl groups are substituted with one or more halogen, thiol, hydroxyl, carbonyl, carboxyl, carbonyloxyl, C1-6alkoxy, C1-6hydroxyalkoxy, amino, C1-6alkylamino, di(C1-6alkyl)amino, azido, piperidinyl, phenyl, naphthyl, pyridyl, pyrimidinyl, imidazolyl, 1,2,3-triazolyl, quinolinyl, isoquinolinyl, thiazolyl, or tetrazolyl groups; and
R4 is hydrogen or halogen;
or is a pharmaceutically acceptable salt thereof.
|