| CPC C07D 265/16 (2013.01) [B64G 99/00 (2022.08); C07D 413/06 (2013.01); C07D 413/14 (2013.01); C08G 73/0233 (2013.01)] | 9 Claims |
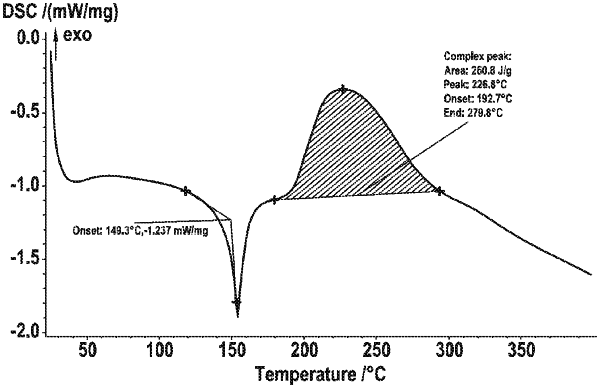
|
1. A process for manufacturing a polybenzoxazine monomer comprising condensing an amine of formula A with a polyaldehyde of formula B in order to obtain the polybenzoxazine monomer of formula C, formulas A, B and C being provided below:
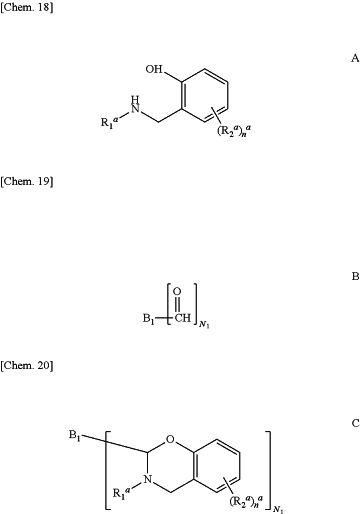 in these formulas:
R1a is a substituted or unsubstituted furfuryl group or a substituted or unsubstituted benzyl group,
R2a is selected from: electron-withdrawing groups; saturated or unsaturated, substituted or unsubstituted, linear or branched hydrocarbon chains comprising between 1 and 6 carbon atoms, interrupted or not interrupted by one or more heteroatoms; saturated, unsaturated or aromatic, substituted or unsubstituted carbocyclic or heterocyclic groups;
na is an integer comprised between 0 and 2;
B1 is selected from: monocyclic or polycyclic, substituted or unsubstituted aromatic carbocyclic or aromatic heterocyclic groups; and
N1 is an integer greater than or equal to 2.
|